Sex, Gender, & Intersectional Analysis
Case Studies
- Science
- Health & Medicine
- Chronic Pain
- Colorectal Cancer
- Covid-19
- De-Gendering the Knee
- Dietary Assessment Method
- Gendered-Related Variables
- Heart Disease in Diverse Populations
- Medical Technology
- Nanomedicine
- Nanotechnology-Based Screening for HPV
- Nutrigenomics
- Osteoporosis Research in Men
- Prescription Drugs
- Systems Biology
- Engineering
- Assistive Technologies for the Elderly
- Domestic Robots
- Extended Virtual Reality
- Facial Recognition
- Gendering Social Robots
- Haptic Technology
- HIV Microbicides
- Inclusive Crash Test Dummies
- Human Thorax Model
- Machine Learning
- Machine Translation
- Making Machines Talk
- Video Games
- Virtual Assistants and Chatbots
- Environment
Animal Research 2: Analyzing How Sex and Extrinsic Lab Conditions Interact
The Challenge
Sex is a fundamental variable that can be used to disaggregate data and explain heterogeneous disease outcomes. Although many factors can influence an outcome, sex is evolutionarily fundamental and impacts the whole of the population. Across diverse disciplines, researchers risk drawing erroneous conclusions when they extrapolate outcome data from one sex to another.
In June 2015, the National Institutes of Health released guidelines for considering sex as a variable in vertebrate animal and human (NIH, 2015; Clayton & Collins, 2014). This follows policies fostering sex/gender analysis in basic research implemented by the Canadian Institutes for Health Research (2010; Johnson et al., 2014) and the European Commission (2013). We expect the US National Science Foundation (NSF) and the European Research Council (ERC) to follow suit in research fields with human endpoints (Schiebinger & Klinge, 2015; 2020; Tannenbaum et al., 2019).
A National Science Foundation-funded workshop was held at Stanford University (September 2014, and updated in 2025) to conceptualize how best to: 1) include male and female animals (primarily rodents) in biomedical research, and 2) analyze potential interactions between extrinsic lab conditions and sex effects in animal research.
Intersectional Innovation 1: Analyzing How Sex and Extrinsic Lab Conditions Interact in Animal Research
Method 1: Analyzing Sex
Method 2: Analyzing How Sex and Lab Environment Interact
The Challenge
Females have been underrepresented in most subfields of animal studies, except reproductive biology and immunology. Importantly, the sex of the animal is not reported in 22–42% of articles in neuroscience, physiology, and interdisciplinary biology journals” (Beery et al., 2011; McCullough et al., 2014). This is research money wasted. If sex is not reported, data cannot be included in meta-analyses.
Intersectional Innovation: Analyzing How Sex and Lab Environment Interact in Animal Research
How can we best design animal studies to take into account sex (biological characteristics) that interact with extrinsic lab conditions (caging practices, sex and behaviors of researchers, room temperature, diet, etc.)? The figure below shows the complex interdependency of sex and lab conditions throughout the rodent life cycle.

Method: Analyzing Sex
- 1. Sex differences must be investigated before they can be ruled out.
- 2. Research can be done stepwise. Male and female animals should be strain- (or strain and genotype) and age-matched, and reared under identical conditions (cages, bedding, diet). Females should not be breeders unless required for assessment of the phenotype.
- Step 1. Total sample size (based on power calculations): Adopting a strategy of both female and male animals or cells seems likely to allow detection of at least some sex influences, namely the largest ones that presumably researchers would first want to detect, with no impact on sample size or cost.
- Step 2. Sex-based powering: tests hypothesis in both males and females and power each to determine effect.
- Step 3. Comparison between sexes: power study to determine the actual “sex effect.” Testing for sex effects has a financial cost. However, a demonstrated sex difference justifies sex-specific research because harm in one sex is costly to society and individual patients. Overall, it is less expensive to understanding sex in the basic science phase than during the more costly clinical trial phase (Freedman et al. 2015; Sertkaya et al. 2024). This may decrease the number of drugs that fail in development and also help companies avoid being forced to remove drugs from the market due to adverse events in one sex.
- 3. To appreciate the presence/absence of sex effects, researchers should also evaluate overlap between groups (similarities between males and females) and difference within groups (differences among males or among females). Overemphasizing sex differences should be avoided.
- 4. Finding no sex effect should also be reported. To reduce publication bias, researchers should report when sex differences (main or interaction effects) are not detected or when data regarding sex differences are statistically inconclusive (Wizemann, 2012). Reporting null results is crucial for meta-analysis.
For phenoytpes that do not display sex difference, future experiments should be sex inclusive, that is include equal numbers of randomly selected males and females for each test group studied. Not every experiment needs to be designed to evaluate sex differences. However, for every experiment, the sex of the animal test subjects should be noted in the article and reported in the methods section to ensure that experiments are reproducible and findings (in one sex) are not over-generalized (to the other sex) (Wizemann, 2012).
Method: Analyzing How Sex and Lab Environments Interact
-
1. Considering the estrous cycle (Byers et al., 2012)
- a. Becker et al. (2005) recommend that researchers record the stage of the estrous cycle: Testing can include two groups of females in two specific stages of the rat estrus cycle or four groups of females, representing the four days of the cycle. Becker et al. caution against testing females on random days of the estrous cycle, because a sex difference that varies with the estrous cycle might be overlooked. They also caution that a small group of females in close contact may synchronize cycles, leading researchers to miss variations that occur with cycles.
- b. In a meta-analysis of nearly 10,000 traits, Prendergast et al. (2014) found that, for most biological measurements, females are no more variable than males. Other factors, including group versus single animal housing, can have a greater impact on variability of a trait than stages of the estrous cycle.
2. Menopause Models
Menopause is an emerging area of research in animal modeling studies. One study reported that immunological changes accompany this hormonal transition. Ovariectomized mice undergo "acute menopause," and exhibit "reduced lymphocyte chemotaxis, mitogen-induced T cell proliferation responses, and [Interleukin-2] production" (Marriott et al., 2006).
3. Pregnancy or Pseudopregnancy
Less than 10% of medications approved by the U.S. Food and Drug Administration since 1980 has enough information to determine risks for birth defects (Adam et al., 2011; Mishra & Mohanty, 2010). New animal research that evaluates drug safety should assess effects on the dam and the fetus during pregnancy and lactation (McDonnell-Dowling & Kelly, 2015).
4. Pharmacokinetics
The estrous cycle can also affect pharmacokinetics. Kulkarni et al. (2012) found that the oral bioavailability of genistein, a soy isoflavone with antioxidant properties, was inversely correlated with estrogen level (which regulates hepatic disposition of a drug).
- 1. When studying a sex-specific phenomenon, such as ovarian cancer or prostate cancer.
- 2. To address inadequate of published data for one sex in a particular area.
- 3. Where there is strong, statistically robust evidence that sex does not influence a trait or outcome.
Animal research includes the interaction between sex (biological characteristics, such as genes, hormones, age, reproductive phase, strain, etc.) and the lab environment (which may include caging practices, attitudes and behaviors of researchers, room temperature, diet, etc.). The double-ended arrows represent interactions between sex and the lab environment.Environmental processes may impact male and female animals differently, such as caging practices or differential handling. Researchers should not identify an effect as dependent on sex (or a biological trait) when, in fact, it depends on an environmental condition.
Extrinsic Lab Conditions that may Interact with Sex Effects Include:
- 1. Caging: Individual vs. group?
- a. To avoid aggressive behaviors, male rodents are often caged in small groups or alone. Rodents housed alone “expend more energy maintaining body temperature, which can cause differences in parameters such as caloric intake, muscle activity, metabolic rate, fat distribution, or body size, with a plethora of potential downstream effects on bodily and cellular activity” (Ritz et al., 2014). In contrast, females are more often housed together to lower costs. Rodents housed together often sleep clustered and, as a result, expend less energy to keep warm. In this scenario a “sex difference” may be identified where, in fact, differences result from different housing conditions.
- b. The same sized group may create different stressors for females and males. Being caged alone may itself cause stress (Ritz et al., 2014), but single housing reduces trait variability in both males and females (Prendergast et al., 2014).
- c. Group caging can also result in self-induced or social hair loss, also called barbering (Kalueuff et al., 2006). Barbering: 1) often reflects social hierarchies in same-sex group cages (both males and females); 2) may result from the stress of overcrowding; 3) takes place in breeding groups (females barber males); and 4) occurs among lactating rodents (pups barber mothers). Barbering occurs in some strains more than others.
- d. Cage size can limit animal behavior. For example, many cages cannot accommodate the full range of female sexual behavior. In the wild, females may dart, approach, and solicit males (Birke, 2011). For these reasons, articles should specify housing conditions, including numbers of animals per cage. Prendergast et al. (2014) found that more than half of their surveyed mouse studies failed to do so.
-
2. Researcher/Staff
Sex of researcher/staff: Experimenters may be a confounding variable in rodent research where stress is a significant factor. One study found that rats and mice demonstrated a reduced pain response in the presence of a male experimenter, as compared with an empty room, whereas the presence of a female experimenter produced no difference. Both male and female rodents showed this response, but females had a greater effect. The researchers identified this “male observer effect” as a stress response to androstenone and androstadienone, axillary secretions found in higher concentrations in males than females. In addition to stress-induced analgesia, the presence of these compounds resulted in increased plasma corticosterone levels (Sorge et al., 2014). -
3. Handling
Control rodents should undergo similarly stressful procedures as those of the experimental rodents, such as sham surgeries. Taking vaginal smears to establish stage of the estrous cycle in female rodents can be stressful; male rodents should be handled in similar ways (Becker et al., 2005). Without these controls differences in stress responses can be mistaken for other sex differences. -
4. Circadian Cycling
Testosterone varies seasonally and with circadian rhythms. Similarly, hormone concentrations in females can fluctuate over the course of a single day of the estrous cycle. Hypothalamic-pituitary-adrenal (HPA) secretions, that in turn affect gonadal secretions, also vary over the course of the day (Becker et al., 2005). Researchers should specify the photoperiod in the colony and the time of day at which measurements are taken. -
5. Social Dynamics
Edelman et al. (2013) found that rat maternal behavior mediates sex differences in play among juveniles. Simulated maternal grooming, in addition to normal maternal care, reduced play in males but not in females. This effect may be mediated by increased serotonin signaling, as maternal licking also increased serotonin receptor mRNA. -
6. Temperature
Laboratory mice are typically housed at temperatures below their thermoneutral zone. Gaskill et al. (2009) found that when mice were able to move among three cages with different temperatures, mice of both sexes preferred warmer environments for inactive and maintenance behaviors (with no preference for active behaviors). Females preferred the highest temperature; males showed no preference between the medium and highest temperatures. As with day length, ambient temperatures in the research colony should be reported consistently. -
7. Diet
Diets impact weight gain, metabolism, hormone levels, and immune functions, hence diet formulation should be reported (Bhupathy et al., 2010; Luczak et al, 2011; Madhusoodanan, 2022). Glover et al. (2006) found that diets rich in phytoestrogens may have sex-specific effects on cardiac health. In males, soy-based diets significantly decreased cardiac function, increased myocellular disarray, and led to an increase in β-MyHC, a myosin motor protein associated with heart failure. This latter effect was also observed to a lesser degree in females. -
8. Behavior
Animal species exhibit substantial variation in behavior that can influence research outcomes. Animal behavior is shaped by genetics, experience, and social background. The STRANGE framework recommends the following factors to improve generalizability and comparison between studies: social background, trappability and self-selection, rearing history, acclimation and habituation, natural changes in responsiveness, genetic make-up, and experience (Webster & Rutz, 2020).
Analyzing sex and lab environments—and how they interact—is important to increasing the translational value of animal models. The cost of developing a drug ranges between $350 million and $5 billion and 95% of drug candidates fail (Arrowsmith 2011; Herper, 2013). Including sex and gender as research variables may help bring down those costs, promote discovery of disease mechanisms, and save lives.
Works Cited
Adam, M., Polifka, J., & Friedman, J. (2011). Evolving Knowledge of the Teratogenicity of Medications in Human Pregnancy. American Journal of Medical Genetics, Part C., 157, 175-182.
Arrowsmith, J. (2011). Trial watch: Phase II Failures: 2008–2010. Nature Reviews Drug Discovery, 10, 328-329.
Becker, JB, Arnold, AP, Taylor, J, Young, E, Berkley, KJ, Blaustein, JD, Eckel, LA, Hampson, E, Herman, JP, Marts, S, Sadee, W, & Steiner, M, (2005). Strategies and Methods for Research on Sex Differences in Brain and Behavior. Endocrinology, 146 (4), 1650-1673.
Beery, A., & Zucker, I. (2011). "Sex Bias in Neuroscience and Biomedical Research." Neuroscience and Biobehavioral Reviews, 35 (3), 565-572.
Bhupathy, P., Haines, C., & Leinwand, L. (2010). Influence of Sex Hormones and Phytoestrogens on Heart Disease in Men and Women. Women's Health 6 (1), 77–95.
Birke, L. (2011). Telling the Rat What to Do: Laboratory Animals, Science, and Gender. Gender and the Science of Difference; Cultural Politics of Contemporary Science and Medicine, ed. Jill A. Fisher. New Brunswick: Rutgers University Press, 91-107.
Byers, S., Wiles, M., Dunn, S., & Taft, A. (2012). Mouse Estrous Cycle Identification Tool and Images. PLoS ONE, 7 (4): e35538. doi:10.1371/ journal.pone.0035538.
Clayton, J. & F. Collins. (2014). NIH to Balance Sex in Cell and Animal Studies. Nature, 509, 282-283.
Edelmann, M. N., Demers, C. H., & Auger, A. P. (2013). Maternal Touch Moderates Sex Differences in Juvenile Social Play Behavior. PLoS ONE, 8 (2), e57386.
Freedman, L. P., Cockburn, I. M., & Simcoe, T. S. (2015). The Economics of Reproducibility in Preclinical Research. PLoS Biology, 13(6), e1002165.
Gaskill, B. N., Rohr, S. A., Pajor, E. A., Lucas, J. R., & Garner, J. P. (2009). Some Like it Hot: Mouse Temperature Preferences in Laboratory Housing. Applied Animal Behavioral Science, 116, 279-285.
Glover A., & Assinder S. J. (2006). Acute Exposure of Adult Male Rats to Dietary Phytoestrogens Reduces Fecundity and Alters Epididymal Steroid Hormone Receptor Expression. Journal of Endocrinology, 189 (3), 565-573.
Herper, M. (2013). The Cost of Creating a New Drug Now $5 Billion, Pushing Big Pharma to Change. Forbes. 8/11/2013.
Johnson J., Sharman Z., Vissandjée, B., Stewart D. (2014). Does a Change in Health Research Funding Policy Related to the Integration of Sex and Gender Have an Impact? PLoS ONE 9(6): e99900.
Kalueff, A., Minasyan, A., Keisala, T., Shah, Z., & Tuohimaa, P. (2006). Hair Barbering in Mice: Implications for Neurobehavioral Research. Behavioral Processes, 71 (1), 8-15.
Klein, S., Schiebinger, L., Stefanick, M., Cahil, L., Danska, J., De Vries, G., Kibbe, M., McCarthy, M., Mogil, J., Woodruff, T., & Zucker, I. (2015). Sex Inclusion in Basic Research Drives Discovery. Proceedings of the National Academy of Science, 112 (17), 5257–5258.
Kulkami K.H., Yang, Z., Niu, T, & Hu, M. (2012). Effects of Estrogen and Estrus Cycle on Pharmacokinetics, Absorption, and Disposition of Genistein in Female Sprague-Dawley Rats. Journal of Agricultural and Food Chemistry, 60, 7949-7956.
Luczack, Elizabeth D., Barthel, Kristen K. B., Stauffer, B. L., Konhilas, John P., Cheung, Tom H., Leinwand, &Leslie A. (2011). Remodeling the Cardiac Transcriptional Landscape with Diet. Physiological Genomics 43, 772-780.
Madhusoodanan, J. (2022). Dietary Differences Can Confound Animal Studies. Nature, 605, 778-779.
Marriott, I., & Huet-Hudson, Y. (2006). Sexual Dimorphism in Innate Immune Responses to Infectious Organisms. Immunologic Research, 34 (3), 177-192.
McCullough, L. D., De Vries, G. J., Miller, V.M., Becker, J. B., Sandberg, K., & McCarthy, M. M. (2014). NIH Initiative to Balance Sex of Animals in Preclinical Studies: Generative Questions to Guide Policy, Implementation, and Metrics. Biology of Sex Differences, 5(1), 1.
McDonnell-Dowling, & Kelly, J. (2015). Sources of Variation in the Design of Preclinical Studies Assessing the Effects of Amphetamine-Type Stimulants in Pregnancy and Lactation. Behavioural Brain Research, 279, 87-99.
Mishra, A. & Mohanty, B. (2010). Effect of Lactational Exposure of Olanzapine on Body Weight of Mice: A Comparative Study on Neonates of Both the Sexes during Post-Natal Development, Journal of Psychopharmacology, 23 (7), 1089-1096.
National Institutes of Health (NIH). (2015). Consideration of Sex as a Biological Variable in NIH-funded Research: http://grants.nih.gov/grants/guide/notice-files/NOT-OD-15-102.html.
Prendergast, B. J., Onishi, K. G. & Zucker, I. (2014). Female mice liberated for inclusion in neuroscience and biomedical research. Neuroscience and Biobehavioral Reviews, 40, 1–5.
Ritz, S., Antle, D., Côté, J., Deroy, K., Fraleigh, N., Messing, K., Parent, L., St-Pierre, J., Vaillancourt C., & Mergler, D. (2014). First Steps for Integrating Sex and Gender Considerations into Basic Experimental Biomedical Research. FASEB J, 28 (1), 4-13. doi: 10.1096/fj.13-233395.
Schiebinger, L & Klinge, I. (2015). Gendered Innovation in Health and Medicine, Gender: Zeitschrift für Geschlecht, Kultur, und Gesellschaft, 2, 29-50. (In English)
Schiebinger, L & Klinge, I. Eds. (2020). Gendered Innovations 2: How Inclusive Analysis Contributes to Research and Innovation (Luxembourg: Publications Office of the European Union, 2020). Foreword by EC Commissioner for Innovation, Research, Culture, Education and Youth, Mariya Gabriel.
Sertkaya, A., Beleche, T., Jessup, A., & Sommers, B. D. (2024). Costs of Drug Development and Research and Development Intensity in the US, 2000-2018. JAMA Network Open, 7(6), e2415445-e2415445.
Sorge, R., Martin, L., Isbester, K., Sotocinal, S., Rosen, S., Tuttle, A., Wieskopf, J., Acland, E., Dokova, A., Kadoura, B., Leger, P., Mapplebeck, J., McPhail, M., Delaney, A., Wigerblad, G., Schumann, A., Quinn, T., Frasnelli, J., Svensson, C, Sternberg, W., & Mogil, J. (2014). Olfactory Exposure to Males, Including Men, Causes Stress and Related Analgesia in Rodents. Nature Methods, 11, 629–632.
Stauffer, Brian L., Konhilas, John P., Luczak, Elizabeth D., Leinwand, & Leslie A. (2006). Soy Diet Worsens Heart Disease in Mice. Journal of Clinical Investigations 116 (1), 209-216.
Tannenbaum, C., Ellis, R. P., Eyssel, F., Zou, J., & Schiebinger, L. (2019). Sex and Gender Analysis Improves Science and Engineering. Nature, 575(7781), 137-146.
Wizemann, T. (Ed.) (2012). Sex-Specific Reporting of Scientific Research: A Workshop Summary. Washington, D.C.: National Academies Press.
Webster, M. M., & Rutz, C. (2020). How STRANGE are your study animals? Nature, 582, 337-340.
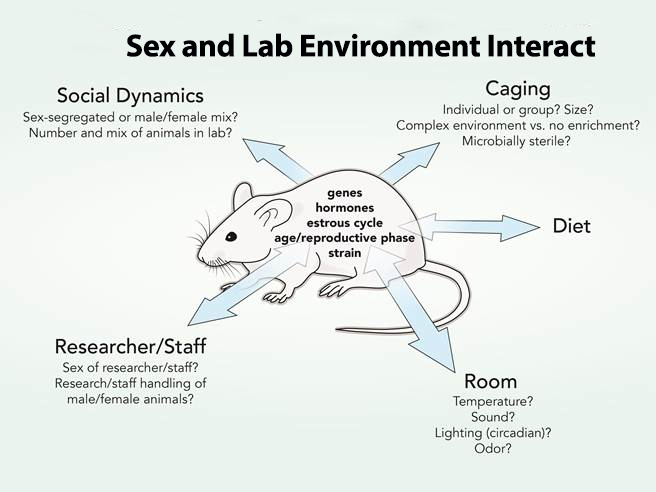 Animal research includes the interaction between sex (biological characteristics, such as genes, hormones, age, reproductive phase, strain, etc.) and cultural or environmental processes (such as caging practices, attitudes and behaviors of researchers, room temperature, diet, etc.). The double-ended arrows represent interactions between the biological sex characteristics of the animal and lab environmental factors. Environmental processes, such as caging practices or differential handling (which may include gender assumptions and practices on the part of researchers), may impact male and female animals differently. Investigators should not identify an effect as dependent on sex (or a biological trait) when, in fact, it is influenced by an environmental (lab) condition.
Animal research includes the interaction between sex (biological characteristics, such as genes, hormones, age, reproductive phase, strain, etc.) and cultural or environmental processes (such as caging practices, attitudes and behaviors of researchers, room temperature, diet, etc.). The double-ended arrows represent interactions between the biological sex characteristics of the animal and lab environmental factors. Environmental processes, such as caging practices or differential handling (which may include gender assumptions and practices on the part of researchers), may impact male and female animals differently. Investigators should not identify an effect as dependent on sex (or a biological trait) when, in fact, it is influenced by an environmental (lab) condition.
One case in point is researcher sex. This example focuses on pain research in the lab. Researchers induce pain in rats and mice. They find that rats and mice don’t show their pain to men researchers. Animals don’t show their pain when a man is in the room, as compared to an empty room, but they do show their pain when a woman is in the room. The researchers identified this as the “male-observer effect.”
What’s going on? It’s not how the researchers act or how they handle the animals. The animals smell the men’s pheromones. According to Jeffrey Mogil at McGill University, this phenomenon may throw into question all prior results from pain research.






