Sex, Gender, & Intersectional Analysis
Case Studies
- Science
- Health & Medicine
- Chronic Pain
- Colorectal Cancer
- Covid-19
- De-Gendering the Knee
- Dietary Assessment Method
- Gendered-Related Variables
- Heart Disease in Diverse Populations
- Medical Technology
- Nanomedicine
- Nanotechnology-Based Screening for HPV
- Nutrigenomics
- Osteoporosis Research in Men
- Prescription Drugs
- Systems Biology
- Engineering
- Assistive Technologies for the Elderly
- Domestic Robots
- Extended Virtual Reality
- Facial Recognition
- Gendering Social Robots
- Haptic Technology
- HIV Microbicides
- Inclusive Crash Test Dummies
- Human Thorax Model
- Machine Learning
- Machine Translation
- Making Machines Talk
- Video Games
- Virtual Assistants and Chatbots
- Environment
Nutrigenomics: Intersectional Approaches
The Challenge
The World Health Organization (WHO) states that non-communicable diseases (NCDs), such as cardiovascular disease, cancers, and diabetes, "are the leading cause of death in the world today" and that modifiable risk factors, such as unhealthy diet, physical inactivity and tobacco use, are responsible for the majority of NCDs (WHO, 2009). The prevalence of these risk factors, however, "varied between country income groups, with the pattern of variation differing between risk factors and with gender" (WHO, 2011). The bases for this variability are multiple and still poorly defined.
Method: Intersectional Approaches
Accounting for gender and other intersecting social factors that make women and men vulnerable to NCDs allows researchers and policy-makers to develop more successful interventions. Analyzing the interaction of sex-specific biological factors and gender-related social factors allows researchers to better understand complex disease patterns and counteract them.
Gendered Innovations:
- a. Sex-Specific Metabolism
- b. Sex-Specific Dietary Responses
- c. Sex-Specific Nutrient Responses
1. Understanding Sex- and Gender-Related Variations in NCDs Risk Factors.
Integrating sex and gender analysis into a life course approach helps researchers understand risks for developing NCDs over time.
2. Determining Sex-Specific Metabolism, Dietary, and Nutrient Responses.
Integrating sex analysis into the field of nutrigenomics provides an understanding of how diets affect females and males at the genetic, molecular and cellular levels.
Gendered Innovation 1: Understanding Sex and Gender-Related Variations in NCDs Risk Factors
Method: Analyzing how Sex and Gender Interact
Method: Intersectional Approaches
Gendered Innovation 2: Determining Sex-Specific Metabolism, Dietary and Nutrient Responses
Method: Analyzing sex
Conclusions
Next Steps
The Challenge
According to the World Health Organization (WHO), "in 2005, 35 million people died from non-communicable diseases (NCDs), which represents 60% of the total number of global deaths in that year. Moreover, between 2005 and 2015, yearly deaths due to NCDs are projected to increase by 17%" (WHO, 2009). Scientists estimate that elimination of modifiable risk factors, such as unhealthy diet, physical inactivity and tobacco use, would prevent "80% of premature heart disease, 80% of premature stroke, 80% of type 2 diabetes and 40% of cancer" (WHO, 2009). The prevalence of these risk factors, however, "varie[s] between country income groups, with the pattern of variation differing between risk factors and with gender" (WHO, 2011). The bases for this variability are multiple and still poorly defined. Gender and sex analysis provide a solid, mechanistic understanding of significant patterns of variation and will be essential to reducing NCDs in the decades to come.
Understanding Sex and Gender-Related Variations in NCDs Risk Factors
NCDs are primarily caused by “preventable risk factors” and are “causally linked with four particular behaviours: tobacco use, physical inactivity, unhealthy diet, and the harmful use of alcohol” (WHO, 2011). Integrating sex and gender analysis into a life course approach can reveal how sex and gender-related factors interact to influence the development of NCDs (Brands et al., 2002). For example, sex-specific biological factors determine responses to particular diets that make women and men more vulnerable to certain types of fat dispersions, and gender-related behaviors result in different levels of accumulated risk for “four key metabolic/physiological changes: raised blood pressure, overweight/obesity, hyperglycemia, and hyperlipidemia” (WHO, 2011). Analyzing various life stages, researchers can determine how sex-specific biological factors and multiple social factors combine to affect the health of women and men (see chart below).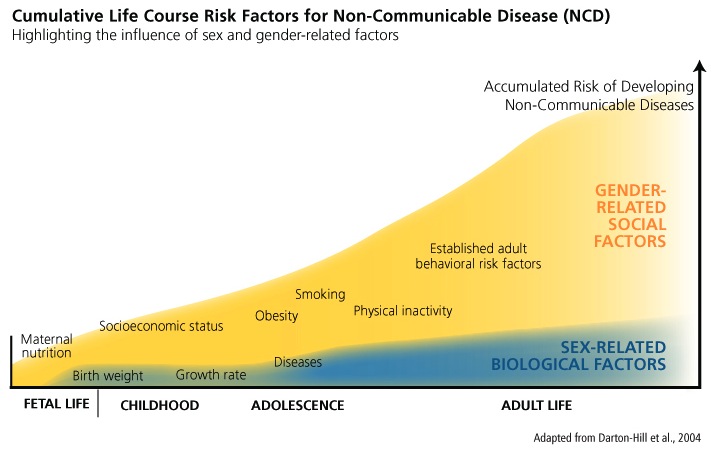
Method: Analyzing How Sex and Gender Interact
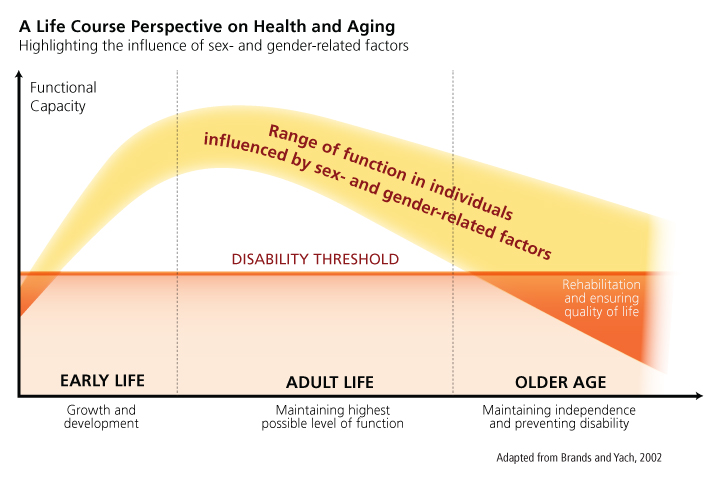
From a life course perspective, the relative influences of sex- and gender-related factors will determine an individual’s functional capacity when aging. It is important to consider that both gender-related social factors and sex-related biological factors interact from early life onwards in the various stages of life, the resulting individual functional capacity is the product of both influences, and therefore it is hard to identify the respective influences of each factor independently.
Promoting healthy behaviors throughout the life course, particularly in adulthood, has been found to prevent the onset of NCDs (see chart below). Several clinical trials and population studies have established that "80% of cases of coronary heart disease (CHD) and up to 90% of type 2 diabetes could potentially be avoided through changing lifestyle factors, and about one third of cancers could be avoided by eating healthily, maintaining normal weight, exercising throughout the life span" (Darnton-Hill et al., 2004). In order to change or modify behaviors, however, the WHO states that it is necessary to examine how "prevailing social and economic conditions influence people's exposure and vulnerability to NCDs" (WHO, 2011). This includes accounting for factors that determine social and economic status, such as "education, occupation, income, gender and ethnicity" (WHO, 2011).
Method: Intersectional Approaches
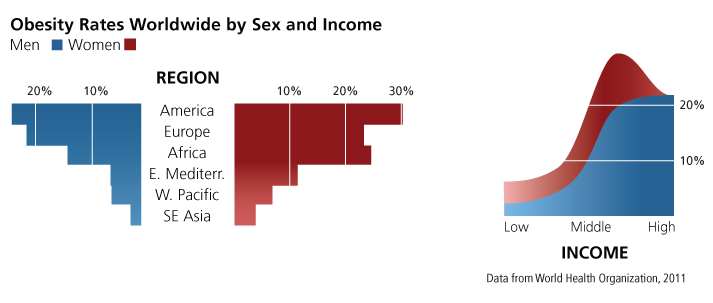
Obesity contributes significantly to the prevalence of NCDs. It has been directly linked to "adverse metabolic effects on blood pressure, cholesterol, triglycerides, and insulin resistance" and increases a person's risk of developing "coronary heart disease, ischemic stroke, and type 2 diabetes mellitus" and "cancer of the breast, colon/rectum, endometrium, kidney, esophagus (adenocarcinoma), and pancreas" (WHO, 2011). Globally, rates of obesity doubled between 1980 and 2008 and are increasing fastest among lower-middle-income countries as a result of modernization on lifestyle and food consumption practices (WHO/FAO, 2003; Popkin et al., 2004).
With the exception of high-income countries where obesity rates are roughly the same for women and men, women are significantly more likely to be obese than men in all regions and income groups (see charts below). There is, however, considerable variation in rates of obesity between women and men across these country groups. For instance, "in low- and lower-middle-income countries, obesity among women was approximately double that among men" (WHO, 2011). Obesity is generally attributed to physical inactivity and poor diet. To understand gender differences in rates of obesity, it is necessary to identify the various social factors that differentially limit women's and men's access to healthy food and physical exercise.
Gendered Innovation 2: Determining Sex-Specific Metabolism, Dietary, and Nutrient Responses
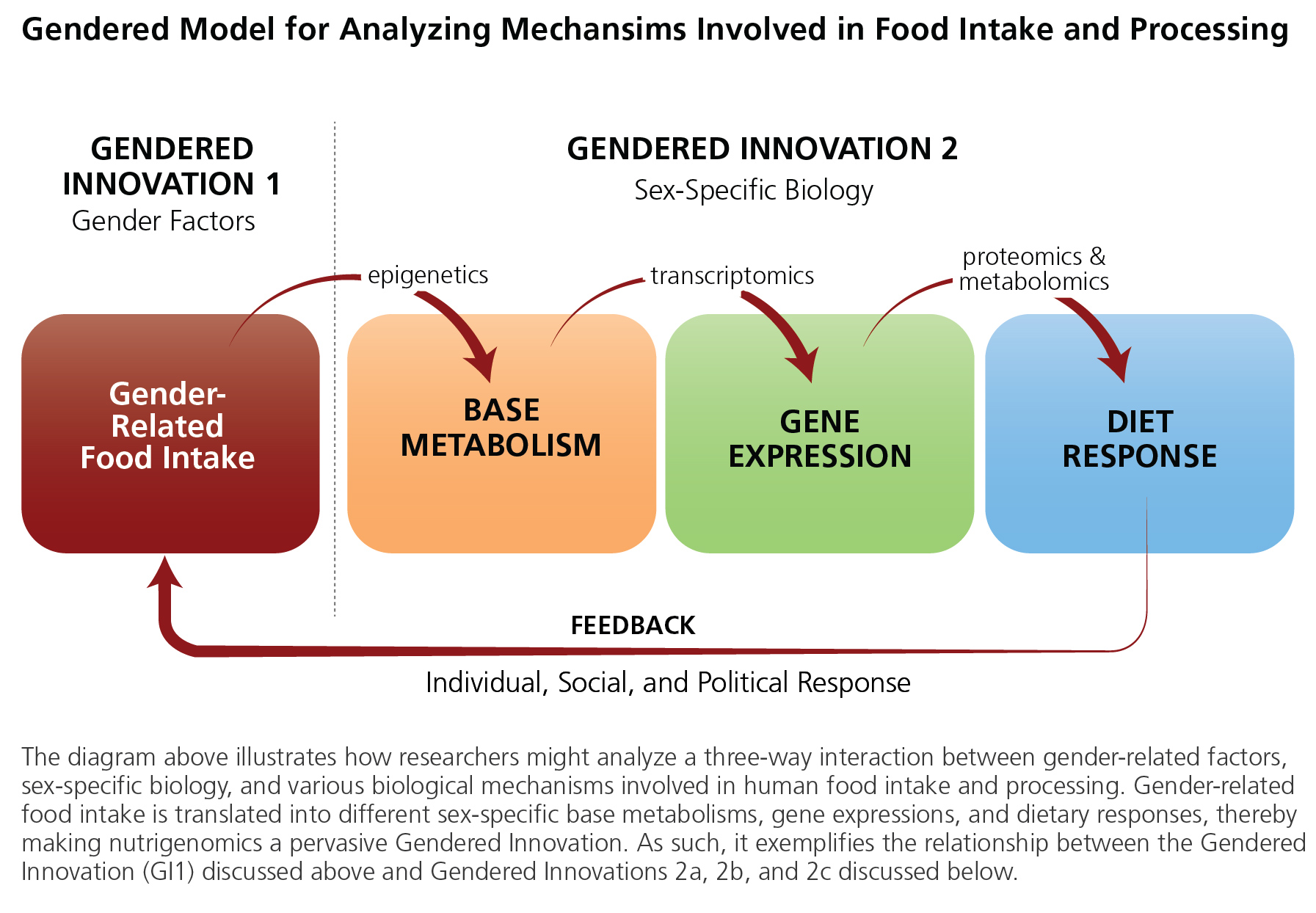
Knowledge of sex differences is essential to complement data from epidemiology in order to understand the biological factors that might be contributing to differences in risk factors, such as obesity. Nutrigenomics "examines the response of individuals to food compounds using post-genomics and related technology" (genomics, transcriptomics, proteomics, metabolomics, etc.). It can be thought of as "the study of how nutrients in food interact with our genes at the molecular and cellular levels, and the impact these reactions have on our health" (Bouwman et al., 2009). Gender-related food intake is a critical part of an individual’s environment and life history. Environment, milieu, and diet are translated into biological variability through the study of epigenetics, analyzing how environmental exposure influences, among other things, metabolism (Niewöhner, 2011). The expectation is that information about an individual's genetic make-up can be combined with knowledge about the biological impacts of environmental context to better assess "personal physical vulnerability to diet-related diseases" (Bouwman et al., 2009).
Method: Analyzing Sex
Dietary recommendations aimed at preventing NCDs are currently a “one size fits all” recommendation. Early studies focused primarily on men. Only in the 1980s did studies begin to include women (i.e., the Nurses’ Health Study, the Minnesota Coronary Survey, and the Finnish Mental Hospital Study) and contradict earlier results. For example, a cohort study of postmenopausal women with established CHD showed that higher saturated fatty acid (SFA) and lower polyunsaturated fatty acid (PUFA) intakes were associated with less progressive coronary atherosclerosis. These results directly contradicted earlier men-only studies that showed an increase in coronary atherosclerosis (Krauss et al., 2000). These new findings launched research into sex differences in basic metabolism, and suggested that biological sex differences in how women and men process nutrients must also be taken into account in prevention strategies.
a. Sex-Specific Metabolism
Serum metabolite concentrations allow a direct readout of biological processes: and association of specific metabolomic signatures with complex diseases (such as Alzheimer’s disease), and cardiovascular and metabolic disorders has been shown. Most studies, however, have not considered the role of sexual dimorphism. Mittelstrass et al. (2011) investigated sex-specific differences of serum metabolite concentrations and their underlying genetic determination. Investigators used more than 3,300 independent individuals from KORA F3 and F4 cohorts with measurement of 131 metabolites, including amino acids, phosphatidylcholines, sphingomyelins, acylcarnitines, and C6-sugars. A linear regression revealed significant concentration differences between men and women for 102 out of 131 metabolites. Sex-Specific Genome Wide Association Studies (GWAS) showed significant differences in beta-estimates for SNPs in the CPS1 locus for glycine. This study indicates that basic metabolite profiles of men and women are significantly different and, furthermore, that specific genetic variants in metabolism-related genes depict sexual dimorphism. Another study analyzing micro-array data of gene expression pointed to sexually dimorphic gene expression in somatic tissue, such as kidney or brain (Isensee et al., 2007). Both studies provide new, important insights into sex-specific differences of cell regulatory processes and underscore that studies should consider sex-specific effects in design and interpretation.
b. Sex-Specific Dietary Responses
Strong mechanistic evidence in support of sex differences in response to dietary intervention comes from animal models using omic-based technologies (i.e., transcriptomics, proteomics). Studies using a rat model found sex-specific plasma protein responses to high-fat diets (Liu et al., 2012; Mukherjee et al., 2012). Another study using animal models found that a greater number of genes encoding myofibrillar proteins and glycolytic proteins were more strongly expressed in males than females when subjects were exposed to a high-fat diet (HFD), reflecting greater muscular activity and higher capacity for using glucose as an energy fuel. But a series of genes involved in oxidative metabolism and cellular defenses were more up-regulated in females than males (Oh et al., 2012). These results suggest that compared to males, females have greater fat clearing capacity in skeletal muscle through the activation of genes encoding enzymes for fat oxidation. Further clinical trials, using sex analysis, are needed to confirm these differences in women and men, but the initial findings suggest that analyzing sex could provide new insights.
c. Sex-Specific Nutrient Responses
Nutritionists using sex analysis have begun to explore—at the functional, mechanistic level—how nutrients affect gene expression and cell function in women and men. For instance, a recent study examined the interplay between inflammation-related genes and vitamin E. Data from a study in 500 elderly nursing home residents were used to examine vitamin E-gene interactions affecting the incidence of respiratory tract infections (RIs). The main finding suggested that the effect of vitamin E on reducing RIs depended on sex. Further research evaluating the effect of vitamin E on RIs should consider both genetic factors and sex, because both were found to have a significant (and interactive) bearing on the efficacy of vitamin E (Belisle et al., 2010).
Conclusions
NCDs now account for the majority of deaths worldwide. By integrating sex and gender analysis into a life course approach, researchers can explore the influence of sex-specific biological factors and gender-related social factors in determining the risk for NCDs. Specifically, analysis of high-risk behaviors indicates that gender attitudes and behaviors promote different patterns of healthy or unhealthy lifestyles among women and men. In addition, recent studies in nutrigenomics document that females and males respond differently to specific diets at the genetic, molecular, and cellular levels. Studies designed to incorporate both sex and gender analysis can provide rich data for designing interventions for healthy living—for researchers, policy makers, and the general public.
Next Steps
1. Information for observational studies (to correlate behavioral and dietary variables, for example) is generally obtained through questionnaires. More research is needed to learn whether women and men provide equally accurate data. Better knowledge of the food consumption will allow researchers to better determine the effects of social environments on various populations and the sex-specific biological outcomes of different patterns of food consumption.
2. Randomized intervention studies designed to investigate female and male responses to specific diets need to include both women and men. Moreover, measurements should include potentially informative biomarkers provided by current omic technologies (e.g., transcriptomics, epigenomics, proteomics and metabolomics), in addition to traditional risk factors.
3. Studies exploring the effects of food consumption should be designed with two time frames in mind: chronic effects (i.e., long-term effects of diets on biomarkers and disease) and acute effects (i.e., postprandial fat challenges, known to be very different between men and women).
Works Cited
Belisle, S.E., Hamer, D.H., Leka, L.S., Dallal, G.E., Delgado-Lista, J., Fine, B.C., Jacques, P.F., Ordovas, J.M., & Meydani, S.N. (2010). L-2 and IL-10 Gene Polymorphisms are Associated with Respiratory Tract Infection and may Modulate the Effect of Vitamin E on Lower Respiratory Tract Infections in Elderly Nursing Home Residents. American Journal of Clinical Nutrition, 92(1), 106-14.
Bouwman, L.I. & Molder, H. (2009). About Evidence and Beyond: A Discourse-Analytic Study of Stakeholders’ Talk on Involvement in the Early Development of Personalized Nutrition. Health Education Research, 24 ( 2), 253-269.
Brands, A. & Yach, D. (2002). NMH Reader Issue no. 1: Women and the Rapid Rise of Non-Communicable Diseases. World Health Organization.
Choi, J.W., Liu, H., Choi, D.K., Oh, T.S., Mukherjee, R., & Yun, J.W.. (2012). Profiling of Gender-specific Rat Plasma Proteins Associated with Susceptibility or Resistance to Diet-induced Obesity. Journal of Proteomics 75 (4), 1386–1400.
Darnton-Hill, I, Nishida, C., & James, W.P. (2004). A Life Course Approach to Diet, Nutrition and the Prevention of Chronic Diseases. Public Health Nutrition, 7 (1a), 101–121.
Isensee, J. & Ruiz Noppinger, P. (2007). Sexually Dimorphic Gene Expression in Mammalian Somatic Tissue. Gender Medicine 4, (Suppl B), S75-S95.
Krauss, R., Eckel, M., Howard, B., Appel, L., Daniels, S., Deckelbaum, R., & Erdman Jr., J. (2000). AHA Dietary Guidelines: Revision 2000: A Statement for Healthcare Professionals from the Nutrition Committee of the American Heart Association. Stroke: A Journal of Cerebral Circulation 31 (11), 2751–2766.
Liu, H., Choi, J.W., & Yun, J.W. (2012). Gender Differences in Rat Plasma Proteome in Response to High-fat Diet. Proteomics 12 (2), 269–283.
Mittelstrass, K., Ried, J., Yu, Z., Krumsiek, J., Gieger, C., Prehn, C., & Roemisch-Margl, W. (2011). Discovery of Sexual Dimorphisms in Metabolic and Genetic Biomarker. PLoS Genetics ,7 (8), e1002215.
Mukherjee, R., Choi, J.W., Choi, D.K., Oh, T.S., Liu, H., & Yun, J.W. (2012). Gender-Dependent Protein Expression in White Adipose Tissues of Lean and Obese Rats Fed a High Fat Diet. Cellular Physiology and Biochemistry, 29 (3-4), 617–634.
Niewöhner, Jörg. (2011). Epigenetics: Embedded Bodies and the Molecularisation of Biography and Milieu. BioSocieties 6 (3) (June 13): 279–298.
Oh, Tae Seok, & Jong Won Yun. (2012). DNA Microarray Analysis Reveals Differential Gene Expression in the Soleus Muscle Between Male and Female Rats Exposed to a High Fat Diet. Molecular Biology Reports, 39 (6) (June): 6569–6580.
Popkin B., & Gordon-Larsen, P. (2004). The Nutrition Transition: Worldwide Obesity Dynamics and their Determinants. International Journal of Obesity and Related Metabolic Disorders, 28 (3), S2-9.
World Health Organization (WHO). (2009). Interventions on Diet and Physical Activity: What Works.
World Health Organization (WHO). (2011). Noncommunicable Diseases: Country Profiles.
WHO/FAO Expert Consultation. (2003). Diet, Nutrition and the Prevention of Chronic Diseases. WHO Technical Report Series 916.
Icon courtesy Keri Piechnik/alive.com
This case examines the epidemic of non-communicable diseases—heart disease, diabetes, and cancers, for example—on the rise across Europe and North America.
Gendered Innovations:
The gendered innovation in this case is understanding sex- and gender-related variables in non-communicable disease risk factors. Integrating sex and gender analysis into a life course approach helps researchers understand risk over time.
1. The diagram (below) shows the relative influences of sex- and gender-related factors determining a person's disease risk over her or his lifetime. Importantly, gender-related social factors (such as obesity, lack of exercise, etc.) interact with sex-related biological factors (such as genetic predispositions, birth weight, and hormones) to determine how a person ages. For instance, to understand differences in women's and men's obesity rates, we need to analyze gender differences in lifestyle. Perhaps gender norms in society lead men to exercise more than women; this can lead to greater disease among women. Or perhaps gender norms in society lead men to eat less healthy food than women. This gendered behavior can lead to greater disease among men.

2. In the figure (below), we see how gender-related food intake can be translated into sex-specific basic metabolism and gene expression, and, finally, into sex-specific responses to dietary interventions. Nutritionists have used sex analysis to explore—at the functional, mechanistic level—how nutrients affect gene expression and cell function in women and men. In one study, they examined vitamin E/gene interactions affecting the incidence of respiratory tract infections in the elderly. The main finding suggested that the effect of vitamin E differed by sex: only in women (with a certain genotype) did vitamin E reduce respiratory tract infections.







