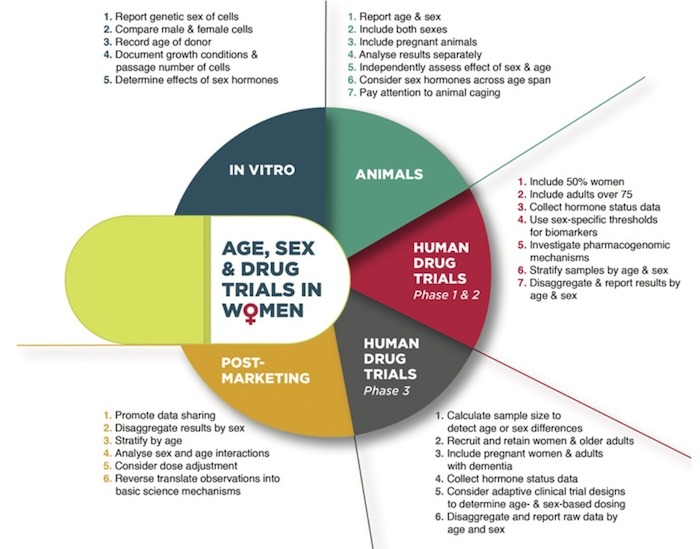
Individualization of drug therapy requires that the right drug be administered at the correct dose to patients who are likely to achieve the highest benefit and lowest risk. Female sex and age comprise two important risk factors for altered drug exposure and response. This review summarizes the current state of science for considering age and sex-related factors along the drug development pipeline, from cell culture and animal research through all phases of clinical trials in humans. A set of recommendations is provided to improve standards for integrating age and sex into the study design, analysis, and reporting of pre-clinical and clinical assessment of new molecular entities and biologics in adults.
Works Cited
For full text, see: Tannenbaum, C., & Day, D. (2017). Age and Sex in Drug Development and Testing for Adults. Pharmacological Research, 121, 83-93, esp. 90. DOI: 10.1016/j.phrs.2017.04.027 Published by Elsevier Ltd. This is an open access article under the CC BY license.





