Author and Reviewer Guidelines for Evaluating Sex, Gender, and Intersectional Analysis in Manuscripts
Editorial boards of peer-reviewed journals can require sophisticated sex, gender, and intersectional analysis when selecting papers for publication to ensure the quality of research. Implementation of such policies has been swift for health and medical journals. The Lancet, for example, adopted such guidelines in December 2016 followed quickly by the International Committee of Medical Journal Editors. Journals in other areas, such as engineering and computer science, are catching up. Journals with policies on sex- and/or gender-specific reporting and journals with policies on race/ethnicity and other socially relevant descriptors are also listed below:
A number of journals have adopted the updated International Committee of Medical Journal Editors (ICMJE) Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals (2016, updated 2022), which state that authors should:
-
• aim for inclusive study populations (age, sex, or ethnicity).
-
• report sex/gender of participants, sex of animals and cells.
-
• describe methods for determining sex/gender.
-
• use sex (biological) and gender (identity, psychosocial, cultural) correctly.
-
• justify use of one sex (unless obvious, such as prostate cancer).
-
• define how they determined race or ethnicity and justify their relevance. In the case where race or ethnicity was not collected, explain why it was not collected. Race and ethnicity are social and not biological constructs; authors should interpret results associated with race and ethnicity in that context.
-
• use neutral, precise, and respectful language to describe study participants.
A number of journals have adopted the ARRIVE guidelines for animal research, which state:
Provide species-appropriate details of the animals used, including species, strain and substrain, sex, age or developmental stage, and, if relevant, weight.
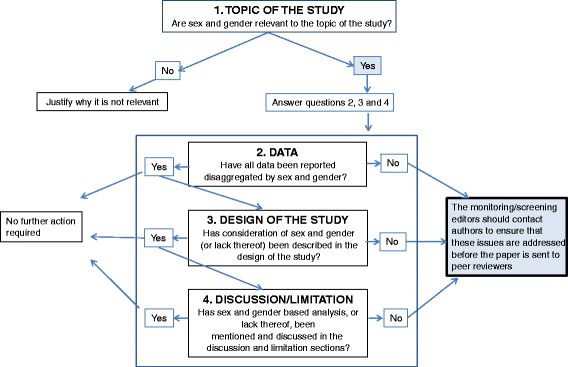
A number of journals have adopted the SAGER guidelines (reproduced at the bottom of this page) published in Research Integrity and Peer Review (2016), which state:
• the title and the abstract of articles should specify the sex /gender of research subjects
• use the words sex and gender carefully
• describe in the methods section how sex was determined (self-report, examination, genetic testing)
• use the term sex in animal studies
• describe the origin and sex of cells or tissues culture in cell biological, molecular biological, or biochemical studies
• where subjects can be differentiated by gender, research should take this into account
• in medical device testing, explain whether a medical device will be used by all genders and if it has been tested with this use in mind
• data should be routinely presented disaggregated by sex
• Sex- and gender-based analyses should be carried out, if appropriate, and reported regardless of positive or negative outcome.
The SAGER checklists for human participants and for other studies (applied sciences, cell biology, etc.) are here.
Journal |
Policy |
| Journal of Studies on Alcohol and Drugs |
JSAD has endorsed the SAGER guidelines. If a study is insufficiently powered to identify sex differences, then this limitation and implications for future research should be discussed.
|
| Journal of the American College of Cardiology |
Author Instructions. |
| American Heart Association/American Stroke Association Journals |
The AHA recommends the NIH Principles and Guidelines for Reporting Preclinical Research, which includes reporting the sex of animals used. The AHA recommends the STROBE guidelines for observational studies and the STARD guidelines for studies diagnostic accuracy, both of which include giving demographic characteristics of participants. Research Guidelines. See also scientific statement from Circulation Vol. 144.
|
| Journal of the American Medical Association |
"Reporting Sex/Gender: The term sex should be used when reporting biological factors and gender should be used when reporting gender identity or psychosocial/cultural factors. The methods used to obtain information on sex, gender, or both (e.g., self-reported, investigator observed or classified, or laboratory test) should be explained in the Methods section. If only one sex is reported, or included in the study, the reason the other sex is not reported or included should be explained in the Methods section, except for studies of diseases/disorders that only affect males (e.g., prostate disease) or females (e.g., ovarian disease).
The sex distribution of study participants or samples should be reported in the Results section, including for studies of humans, tissues, cells, or animals. Study results should disaggregate and report all outcome data by sex. (Journal of the American Medical Association Network). |
| American Journal of Physiology (AJP) |
Report the sex and/or gender, age range, and race of participants. Editorial Policies. See section on Sex and Gender in Study Design. |
| American Journal of Preventative Medicine |
“For research involving or pertaining to humans, animals or eukaryotic cells, investigators should integrate sex and gender-based analyses (SGBA) into their research design according to funder/sponsor requirements and best practices within a field. Authors should address the sex and/or gender dimensions of their research in their article. In cases where they cannot, they should discuss this as a limitation to their research's generalizability. Importantly, authors should explicitly state what definitions of sex and/or gender they are applying to enhance the precision, rigor and reproducibility of their research and to avoid ambiguity or conflation of terms and the constructs to which they refer (see Definitions section below). Authors can refer to the Sex and Gender Equity in Research (SAGER) guidelines and the SAGER guidelines checklist. These offer systematic approaches to the use and editorial review of sex and gender information in study.”
Author Instructions and Editorial Policies
|
| British Journal of Pharmacology (BJP) |
BJP “requires sex to be considered as an experimental variable for all experimental reporting.” See Sex: A Change to Our Guidelines to Authors to Ensure This is No Longer an Ignored Experimental Variable."
|
| BMJ Global Health |
We encourage the use of the Sex and Gender Equity in Research (SAGER) guidelines for reporting of sex and gender information in study design, data analyses, results and interpretation. This includes the correct use of the terms sex (when reporting biological factors) and gender (when reporting identity, psychosocial, or cultural factors) and separate reporting and interpretation of the data by sex and gender. If sex and/or gender information are not reported, this should be explained. See SAGER guidelines and Sex and gender reporting in global health: new editorial policies. BMJ author guidelines
|
| Canadian Medical Association Journal |
Endorses ICMJE recommendations: Submission Guildlines
|
| Cell Press/The Lancet |
Reporting sex- and gender-based analyses (SGBA)
For research involving or pertaining to humans, animals, model organisms, or eukaryotic cells, investigators should integrate sex- and gender-based analyses (SGBA) into their research design according to funder/sponsor requirements and best practices within a field. Authors should address their research's sex and/or gender dimensions in their manuscript. In cases where they cannot, they should discuss this as a limitation to their research's generalizability. With research involving cells and model organisms, researchers should use the term "sex." With research involving humans, researchers should consider which terms best describe their data (see "definitions" section below). Authors can refer to the Sex and Gender Equity in Research (SAGER) Guidelines and the SAGER guidelines checklist. They offer systematic approaches to the use and editorial review of sex and gender information in study design, data analysis, outcome reporting, and research interpretation. However, there is no single, universally agreed-upon set of guidelines for defining sex and gender or reporting SGBA.
Definitions
In human research, the term "sex" carries multiple definitions. It often refers to an umbrella term for a set of biological attributes associated with physical and physiological features (e.g., chromosomal genotype, hormonal levels, or internal and external anatomy). It can also signify a sex categorization, most often designated at birth ("sex assigned at birth") based on a newborn's visible external anatomy. The term "gender" generally refers to socially constructed roles, behaviors, and identities of women, men, and gender-diverse people that occur in a historical and cultural context and might vary across societies and over time. Gender influences how people view themselves and each other, how they behave and interact, and how power is distributed in society. Sex and gender are often incorrectly portrayed as binary (female or male; woman or man), concordant, and static. However, these constructs exist along a spectrum that includes additional sex categorizations and gender identities, such as people who are intersex/have differences of sex development (DSD) or identify as non-binary. In any given person, sex and gender might not align, and both can change. Sex and gender are not entirely discrete concepts, and their definitions continue to evolve. Biology and society influence both, and many languages do not distinguish between them. Since the terms "sex" and "gender" can be ambiguous, authors should describe the methods they use to gather and report sex- and/or gender-related data (e.g., self or physician report, specific biological attributes, current sex or gender, sex assigned at birth, etc.) and discuss the potential limitations of those methods. This will enhance the research's precision, rigor, and reproducibility and help to avoid ambiguity or conflation of terms and the constructs to which they refer. Authors should use the term "sex assigned at birth" rather than "biological sex," "birth sex," or "natal sex," as it is more accurate and inclusive. When asking about gender and sex, researchers should use a two-step process: (1) ask for gender identity allowing for multiple options and (2) if relevant to the research question, ask for sex assigned at birth. In addition to this defining guidance and the SAGER guidelines, you can find further information about reporting sex and gender in research studies in Elsevier's diversity, equity, and inclusion in publishing author guide available here.
|
| Clinical Orthopaedic and Related Research |
For clinical research, authors should include relevant demographic data of the study population, including age, gender/sex distribution, BMI. Reviewer Tools (See Instructions for Using Clinical Research Article Building Tool.) A 2014 editorial recommends the following, but notes that they are not a requirement of publishing in CORR®:
• Design studies that are sufficiently powered to answer research questions both for males and females (or men and women) if the health condition being studied occurs in both sexes/genders.
• Provide sex- and/or gender-specific data where relevant in all clinical, basic science, and epidemiological studies.
• Analyze the influence (or association) of sex or gender on the results of the study, or indicate in the Patients and Methods section why such analyses were not performed, and consider this topic as a limitation to cover in the Discussion section. Readers need to know whether the results generalize to both sexes/genders.
• Indicate (if sex or gender analyses were performed post-hoc) that these analyses should be interpreted cautiously because they may be underpowered (leading to a false conclusion of no difference). If there are many such analyses, indicate that they may lead to spurious significance, and an erroneous conclusion of a sex- or gender-related difference.
|
| Elsevier |
In 2023, Elsevier updated their Guide for Authors to include guidelines for “Sex- and Gender-Based Analysis” in manuscripts across ~2,300 journals in their portfolio (excluding only a few journals in mathematics and physics). These guidelines apply across fields where they were not previously requested, including:
• Information and Software Technology https://www.elsevier.com/journals/information-and-software-technology/0950-5849/guide-for-authors
• Engineering Failure Analysis https://www.elsevier.com/journals/engineering-failure-analysis/1350-6307/guide-for-authors
• Environmental Development https://www.elsevier.com/journals/environmental-development/2211-4645/guide-for-authors
• Journal of Molecular Spectroscopy https://www.elsevier.com/journals/journal-of-molecular-spectroscopy/0022-2852/guide-for-authors
• Artificial Intelligence https://www.elsevier.com/journals/artificial-intelligence/0004-3702/guide-for-authors
The guidelines read: Reporting Sex- and Gender-Based Analyses
Reporting guidance
For research involving or pertaining to humans, animals or eukaryotic cells, investigators should integrate sex and gender-based analyses (SGBA) into their research design according to funder/sponsor requirements and best practices within a field. Authors should address the sex and/or gender dimensions of their research in their article. In cases where they cannot, they should discuss this as a limitation to their research's generalizability. Importantly, authors should explicitly state what definitions of sex and/or gender they are applying to enhance the precision, rigor and reproducibility of their research and to avoid ambiguity or conflation of terms and the constructs to which they refer (see Definitions section below). Authors can refer to the Sex and Gender Equity in Research (SAGER) guidelines and the SAGER guidelines checklist. These offer systematic approaches to the use and editorial review of sex and gender information in study design, data analysis, outcome reporting and research interpretation - however, please note there is no single, universally agreed-upon set of guidelines for defining sex and gender.
|
| Journal of the Endocrine Society |
Reporting the Sex of Human Research Subjects
The sex of research subjects must be indicated.
• If both males and females were included in the study, the numbers of subjects from each sex must be indicated, and it must be indicated whether sex was considered a factor in the statistical analysis of the data.
• Likewise, the sex from which human primary cell cultures or human tissues were obtained must be indicated.
• The authors are also encouraged to include the sex of human cell lines.
Reporting the Sex of Research Animals
• Where applicable, the strain and sex of animals used in research studies must be indicated.
• If both males and females were used, the numbers of animals from each sex must be indicated, and it must be indicated whether sex was considered a factor in the statistical analysis of the data.
• Likewise, the sex from which primary cell cultures or tissues were obtained must be indicated.
• The authors are also encouraged to include the sex of cell lines.
Author guidelines. J. Blaustein, Animals Have a Sex, and so Should Titles and Methods Sections of Articles in Endocrinology.
|
Experimental Physiology and the Journal of Physiology
|
Endorses ARRIVE guidelines for animal experiments (report sex).
Authors should also state the sex of human participants The terms 'gender' and 'sex' should not be used interchangeably.
) |
| Journal of the International AIDS Society |
"Submitting authors should include data disaggregated by sex (and, whenever possible, by race or ethnicity) and provide a comprehensive analysis of gender and racial or ethnic differences. The authors should include the number and percentage of men, women and, if appropriate, transgender persons, who participated in the research study. Anatomical and physiological differences between men and women (height, weight, body fat-to-muscle ratios, cell counts, hormonal cycles, etc.), as well as social and cultural variables (socio-economic, education, access to care, etc.), should be taken into consideration in the presentation of data and/or analysis of the results. Author Instructions. |
| Journal of the Faculty of Medicine (Revista de la Facultad de Medicina Humana) |
The Journal of the Faculty of Medicine endorses the SAGER guidelines. |
| Journal of Korean Medical Sciences |
Ensure correct use of the terms sex (when reporting biological factors) and gender (identity, psychosocial or cultural factors), and, unless inappropriate, report the sex or gender of study participants, the sex of animals or cells, and describe the methods used to determine sex or gender. If the study was done involving an exclusive population, for example in only one sex, authors should justify why, except in obvious cases (e.g., prostate cancer). Authors should define how they determined race or ethnicity and justify their relevance.
|
| Journal of the National Cancer Institute |
“The Journal follows the precepts of the International Committee of Medical Journal Editors (see "ICMJE Recommendations".)” |
| Nature |
Studies Involving Animals and Human Research Participants
Sex and other characteristics of animals that may influence results must be described.
Sex, Gender (Identity/Presentation), and Sexual Orientation
Researchers are encouraged to follow the Sex and Gender Equity in Research – SAGER – guidelines and to include sex and gender considerations where relevant (overview can be found here). We recommend consulting the full guidelines when designing research studies and before submission. These guidelines apply to studies involving humans, vertebrate animals and cell lines.
Authors should use the terms sex (biological attribute) and gender (shaped by social and cultural circumstances) carefully in order to avoid confusing both terms.
The following recommendations and requirements (adapted from the SAGER guidelines) will apply to studies under consideration at Nature journals (including Nature & Nature Communications), Communications Journals and Nature Partner Journals involving human participants and vertebrate animals, where relevant to the topic of study. From June 2022 onwards, Nature Cancer, Nature Communications, Nature Medicine and Nature Metabolism will introduce a pilot actively encouraging authors to report on points (i)-(iii) below. We also urge responsible communication of research findings on sex and gender differences so as to avoid inadvertent perpetuation of harmful gender stereotypes.
i. Title and/or abstract should indicate when the findings apply to only one sex or gender
ii. Describe in the Nature Portfolio Reporting Summary whether sex and gender were considered in the study design, whether sex and/or gender of participants was determined based on self-report or assigned (and methodology used).
iii. Data should be reported disaggregated for sex and gender where this information has been collected and consent has been obtained for reporting and sharing individual-level data; disaggregated numbers for individual experiments must be provided in the source data as appropriate whereas overall numbers may be provided in the Nature Portfolio Reporting Summary.
iv. If sex- and gender-based analyses have been performed a priori, results should be reported regardless of positive or negative outcome. Authors should refrain from conducting post hoc sex- and gender-based analysis if the study design is insufficient (for example, low sample size) to enable meaningful conclusions.
v. If no sex- and gender-based analyses have been performed, authors should justify reasons for lack of analysis in the Nature Portfolio Reporting Summary.
Race, Ethnicity, or other Socially Relevant Groupings in Research
Please specify the socially constructed or socially relevant categorization variable(s) used in your manuscript and explain why they were used. Please note that such variables should not be used as proxies for other socially constructed/relevant variables (for example, race/ethnicity should not be used as a proxy for socioeconomic status). Provide clear definitions of the relevant terms used, how they were provided (by the participants/respondents, the researchers, or third parties), and the method(s) used to classify people into the different categories (e.g., self-report, census, or administrative data, social media data, etc.) Please provide details about how you controlled for confounding variables in your analyses.
Why Nature is updating advice to authors on reporting race and ethnicity
Nature Portfolio Reporting Summary.
For more information, see Nature, Research on Human Populations (including reporting standards).
|
Nature Cancer
Nature Communication
Nature Medicine
Nature Metabolism |
From June 2022 onwards, Nature Cancer, Nature Communications, Nature Medicine and Nature Metabolism will introduce a pilot actively encouraging authors to report on points (i)-(iii) below. We also urge responsible communication of research findings on sex and gender differences so as to avoid inadvertent perpetuation of harmful gender stereotypes.
i. Title and/or abstract should indicate when the findings apply to only one sex or gender
ii.describe in the Nature Portfolio Reporting Summary whether sex and gender were considered in the study design, whether sex and/or gender of participants was determined based on self-report or assigned (and methodology used).
iii. data should be reported disaggregated for sex and gender where this information has been collected and consent has been obtained for reporting and sharing individual-level data; disaggregated numbers for individual experiments must be provided in the source data as appropriate whereas overall numbers may be provided in the Nature Portfolio Reporting Summary. See: https://www.nature.com/nature-portfolio/editorial-policies/ethics-and-biosecurity
|
Journal of Neuroscience Research
|
Addressing Sex as a Biological Variable:
The National Institutes of Health now mandates the inclusion of sex as a biological variable. To conform with this mandate, JNR has established new policy requiring all authors to ensure proper consideration of sex as a biological variable.
These are as follows:
1. Any paper utilizing subjects (cells, animals, humans) of only one sex must state the sex of the samples in the title and abstract of the paper, with the obvious exception of sex-specific issues (e.g., prostate or ovarian function). Authors must also state the rationale for using samples from one sex rather than from both.
2. All papers must clearly state in the methods section the number of samples/subjects of each sex used in the research. For cellular work, the sex of origin of cells used should be reported in most cases. If cells or tissue from both sexes were used without regard to sex, this fact should be indicated.
3. JNR is particularly interested in experiments involving both male and female subjects studied at the same time, and with sufficient sample size to ensure meaningful statistical comparisons. The inability for any reason to study sex differences where they may exist should be discussed as a study limitation.
4. Manuscripts reporting exploratory analyses of potential sex differences in studies not explicitly designed to address them are encouraged. JNR understands the real risk of false-positive errors associated with subgroup analysis, but that risk is balanced by the equal or greater risk of false-negative errors resulting from a failure to consider possible sex influences. JNR also understands that false negative results may result from underpowered analyses, but given the dearth of such analyses in neuroscience to date, and the now clear imperative to change the status quo on this issue, explicitly exploratory analyses are called for in many circumstances.
5. Clinical work should be designed with stratified randomization by sex. Post hoc analyses may also be useful, again perhaps explicitly designated as exploratory.
Editorial: E. Prager, "Addressing Sex as a Biological Variable."
|
| Nordic Studies on Alcohol and Drugs |
Nordic Studies on Alcohol and Drugs endorses the SAGER guidelines.
|
| PLoS Biology/Medicine |
For studies involving humans categorized by race/ethnicity, age, disease/disabilities, religion, sex/gender, sexual orientation, or other socially constructed groupings, authors should:
• Explicitly describe their methods of categorizing human populations
• Define categories in as much detail as the study protocol allows
• Justify their choices of definitions and categories, including for example whether any rules of human categorization were required by their funding agency
• Explain whether (and if so, how) they controlled for confounding variables such as socioeconomic status, nutrition, environmental exposures, or similar factors in their analysis
In addition, outmoded terms and potentially stigmatizing labels should be changed to more current, acceptable terminology. Examples: “Caucasian” should be changed to “white” or “of [Western] European descent” (as appropriate); “cancer victims” should be changed to “patients with cancer.”
|
| Sexual and Reproductive Health Matters |
The SRHM journal recommends the Sex and Gender Equity in Research (SAGER) guidelines. If authors have not disaggregated data by sex, they should provide a justification.
|
| Science |
Authors should report the sex of animals and the gender of human subjects. Editorial Polices
|
| Journal of Surgical Research |
Subscribes to ICMJE recommendations. For animal experiments, the journal endorses the ARRIVE guidelines. Author Information
|
| Surgery |
“For animal experiments, the sex of animal used must be indicated. If both males and females were used, the number of animals from each sex must be indicated, and it must be indicated whether the sex of animal was considered a factor in the statistical analysis of the data. If only one sex was used for the animal studies, the rationale for using only one sex must be indicated. For cell culture experiments, the sex from which primary cell cultures or tissues were obtained must be indicated. The authors are also encouraged to include sex of cell lines. If cells or tissues from both sexes were used without regard to sex, this should be indicated.” Guide for Authors. July 2018 Editorial
|
| Wiley |
Wiley's Best Practice Guidelines on Research Integrity and Publishing Ethics is an updated, living document that provides information and guidance to support all involved in scholarly publishing. It is written for researchers, in their various roles as editors, authors and peer reviewers; societies; librarians; funders; corporations; publishers; and journalists. It includes a summary of best practices with respect to research integrity and publishing ethics from leading organizations in the field: 1) ARRIVE guidelines for animal research amongst other relevant guidance, 2) ICMJE recommendations for human studies, including guidance on bias-free language, 3) Sex and Gender Equity in Research (SAGER) guidelines for both animal and human studies, and 4) COPE for principles of research ethics, including The Principles of Transparency and Best Practice in Scholarly Publishing. Wiley is also a signatory of the Joint Commitment for Action Inclusion and Diversity in Scholarly Publishing and we encourage researchers and editors to learn about and adopt the diversity, equity, inclusion principles and practices it recommends. We also recommend the toolkits produced by the Coalition for Diversity and Inclusion in Scholarly Communication (C4DISC).
|
General principles
• Authors should use the terms sex and gender carefully in order to avoid confusing both terms.
• Where the subjects of research comprise organisms capable of differentiation by sex, the research should be designed and conducted in a way that can reveal sex-related differences in the results, even if these were not initially expected.
• Where subjects can also be differentiated by gender (shaped by social and cultural circumstances), the research should be conducted similarly at this additional level of distinction.
Recommendations per section of the article
| Title and Abstract | If only one sex is included in the study, or if the results of the study are to be applied to only one sex or gender, the title and the abstract should specify the sex of animals or any cells, tissues and other material derived from these and the sex and gender of human participants. |
| Introduction | Authors should report, where relevant, whether sex and/or gender differences may be expected. |
| Methods | Authors should report how sex and gender were taken into account in the design of the study, whether they ensured adequate representation of males and females, and justify the reasons for any exclusion of males or females. |
| Results | Where appropriate, data should be routinely presented disaggregated by sex and gender. Sex- and gender-based analyses should be reported regardless of positive or negative outcome. In clinical trials, data on withdrawals and dropouts should also be reported disaggregated by sex. |
| Discussion | The potential implications of sex and gender on the study results and analyses should be discussed. If a sex and gender analysis was not conducted, the rationale should be given. Authors should further discuss the implications of the lack of such analysis on the interpretation of the results.
|
SAGER flowchart guiding editors' initial screening of submitted manuscripts






