Sex, Gender, & Intersectional Analysis
Case Studies
- Science
- Health & Medicine
- Chronic Pain
- Colorectal Cancer
- Covid-19
- De-Gendering the Knee
- Dietary Assessment Method
- Gendered-Related Variables
- Heart Disease in Diverse Populations
- Medical Technology
- Nanomedicine
- Nanotechnology-Based Screening for HPV
- Nutrigenomics
- Osteoporosis Research in Men
- Prescription Drugs
- Systems Biology
- Engineering
- Assistive Technologies for the Elderly
- Domestic Robots
- Extended Virtual Reality
- Facial Recognition
- Gendering Social Robots
- Haptic Technology
- HIV Microbicides
- Inclusive Crash Test Dummies
- Human Thorax Model
- Machine Learning
- Machine Translation
- Making Machines Talk
- Video Games
- Virtual Assistants and Chatbots
- Environment

Marine Science: Analyzing Sex
The Challenge
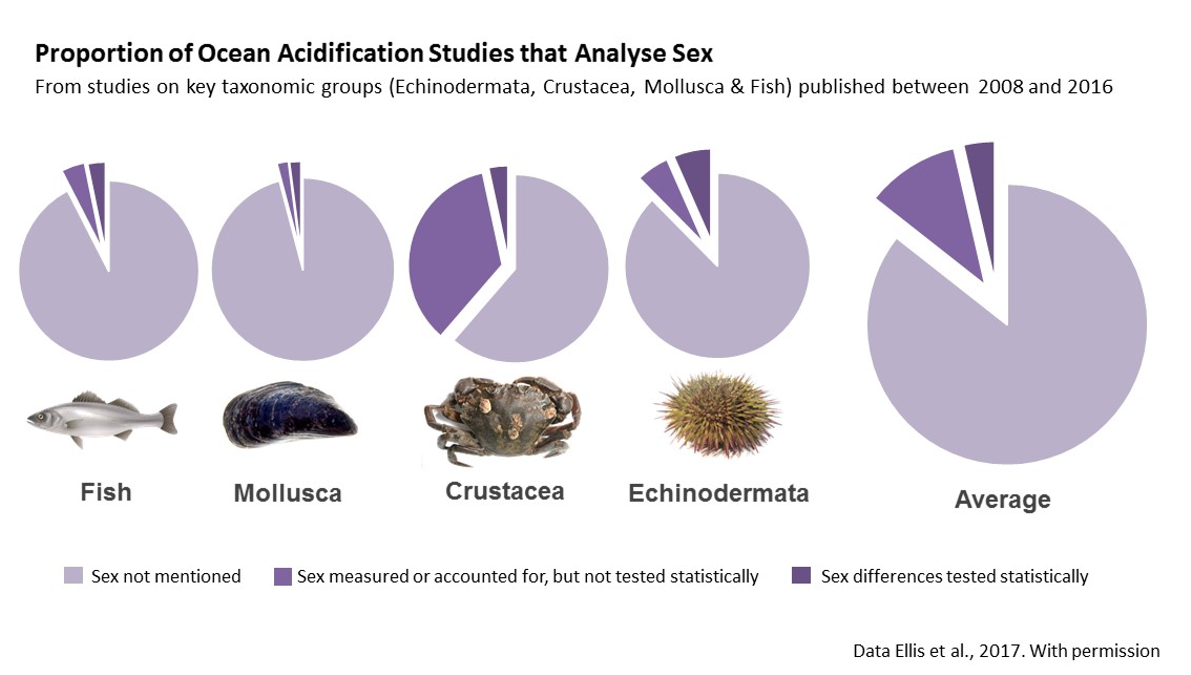
Sex analysis is largely overlooked in marine science (Tannenbaum et al., 2019). A recent systematic review of ocean acidification literature, for example, revealed that only 3.7% of recent studies tested for sex differences, and 85% of studies failed to consider sex at all (Ellis et al., 2017). Failing to account for such differences impacts our ability to effectively manage ecosystems and set conservation priorities.
Methods: Rethinking Concepts and Theories:
The inclusion of sex in marine science requires challenging the widely held perception that sex plays no role in determining biological responses to environmental disturbances. We must also move beyond the assumption that sex is binary, fixed, and genetically determined. Nature abounds with examples of sex along a continuum, labile sex, and sex determined by an interacting suite of genetic, hormonal, physiological, social and/or environmental processes. In order to understand the importance of sex for basic biology, it is important to consider the mechanisms, timings, and direction of sex determination and differentiation, as well as differences in female, male and hermaphroditic responses to environmental perturbation.
Methods: Analyzing Sex:
Studies that disaggregate and analyze data by sex have highlighted the importance of considering female and male responses to climate change. In many marine organisms, sex cannot be easily seen. What is needed now is novel, non-invasive methods of sex analysis in non-model organisms, an effort that will be facilitated by the increasing affordability of ‘omics’ approaches.
Gendered Innovations:
1. Understanding Feminization in a Warming Ocean is critical to elucidate the threat that climate change poses to animals whose sex is determined by temperature, such as in marine turtles. Understanding such phenomena is vital to assess extinction risk under future climate change scenarios.
2. Understanding Environmental Sex Determination beyond Temperature is vital to population demography in a number of organisms of economic, ecological and experimental importance to humans under future climate change scenarios.
3. Effective Population Management via Sex Analysis will enable continued sustainable exploitation of natural resources.
4. Understanding the Differential Sensitivity of Females and Males to Climate Change is critical to fully documenting the impact of environmental change on populations and ecosystems.
The Challenge
Anthropogenically induced climate change is one of the most serious and pervasive global challenges (Hoegh-Guldberg & Bruno, 2010; Diffenbaugh & Field, 2013). To minimize adverse outcomes, it is critical to establish and enforce effective ecosystem management (Hoegh-Guldberg & Bruno, 2010), which in turn depends on a robust understanding of organism and ecosystem resilience to environmental change (O’Leary et al., 2017; Patricio et al., 2019). We do not yet sufficiently understand the extent to which such resilience varies with sex.
The sex ratio of a population is a main determinant of its resilience to environmental disturbance (Ospina-Alvarez & Piferrer, 2008). Sex ratios are driven in part by differential sensitivity of females, males and hermaphrodites to climate change stressors. Nonetheless, sex is rarely analyzed as an experimental variable (Tannenbaum et al., 2019). A recent systematic review of ocean acidification literature, for example, revealed that only 3.77% of recent studies tested for sex-based differences (see figure below), while 85% of studies failed to consider sex at all (Ellis et al., 2017). This results in a lack of experimental evidence on which to assess sex-specific impacts of ocean acidification, limiting our ability to accurately appraise the resilience of marine populations to reduced seawater pH. Where tested, clear sex-based differences to elevated pCO2 have been documented (Ellis et al., 2017). Failing to account for such differences impacts our ability to effectively manage ecosystems and to determine conservation priorities.
Method: Rethinking Concepts and Theories
A major barrier to the inclusion of sex as an experimental variable is the widely held perception that sex is unimportant for interpreting species responses to changing environmental conditions. In many instances, such perceptions stem from the lack of documented sex differences in organisms beyond traditional model-organisms, such as C. elegans. Challenging such perceptions requires an integrated approach across all three pillars of academic research: policy, publication and education (Tannenbaum et al., 2019). Funding agency policies can drive increased sex-based research in non-model organisms. This increase in research and subsequent peer-reviewed literature will provide key examples to the next generation of experimentalists of how to successfully implement robust methods of sex and gender analysis in relevant areas of research.
A further widely held assumption across both science and society is that sex is binary, genetically determined, and fixed at fertilization. Exceptions to this rule are often considered anomalous (Bachtrog et al., 2014). Across nature, however, diverse sexual forms and mechanisms of sex determination challenge these norms and support instead an understanding of a sex continuum (Ainsworth, 2015). Hermaphroditism (the presence of male and female gametes in the same individual), for example, occurs in 94% of flowering plants (Renner & Ricklefs, 1995) and in 1 in 3 species of non-insect animals (Jarne & Auld, 2006). When hermaphroditism is sequential (an individual changes from one distinct sex to another during its lifetime—in some species on more than one occasion), sex is shown to be a labile trait (Munday et al., 2006). Even if distinct female and male sexual forms become clear, unambiguous and fixed, sex determination often depends on an interacting suite of genetic, hormonal, physiological, social and/or environmental processes. In order to understand the importance of sex for basic biology, it is important to consider the mechanisms, timings and direction of sex determination and differentiation, as well as differences in female, male and hermaphroditic responses to environmental perturbation (Ellis et al., 2017; Tannenbaum et al., 2019).
Method: Analyzing Sex
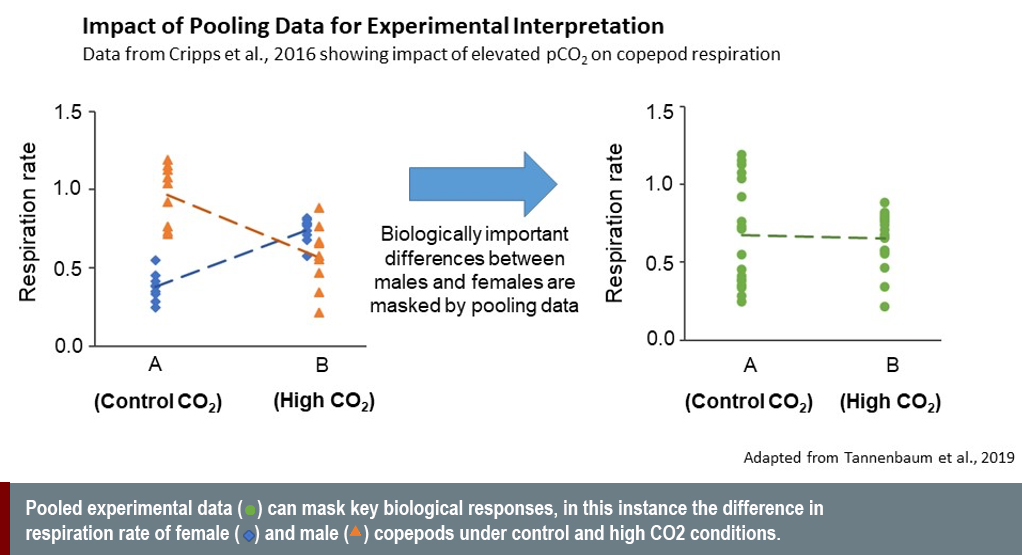
Disaggregating experimental results by sex reveals biologically significant differences that would otherwise be masked if data were pooled (see graph below). To do this, however, requires accurate sexing techniques (and where possible non-invasive techniques). The recent development of novel methods for sexing individuals in non-model marine organisms, which include genetic (Benestan et al., 2017; Hines et al., 2007), metabolomic (Ellis et al., 2014; Hines et al 2007) and endocrine techniques (Jensen et al., 2018), has significantly enhanced our ability to accurately project climate change impacts in certain groups. But concerted efforts to expand these approaches to wider groups of marine organisms are vital. These efforts will be advanced through the continued innovation and increased affordability of omics approaches.
Gendered Innovation 1: Understanding Feminization in a Warming Ocean
Temperature-dependent sex determination (TSD) in marine turtles is perhaps the most widely studied and well-known mechanism of environmental sex determination in nature. However, species that exhibit TSD are also widely considered among the most vulnerable to climate change given the possibility that increasing temperatures favor the production of one sex over the other, destabilizing population sex ratios (Mitchell & Janzen, 2010). In marine turtles, sex is determined by temperature during egg incubation, with higher temperatures resulting in a greater proportion of females (Hawkes et al., 2007; Hawkes et al., 2009; Patricio et al., 2019).
Studies have projected highly female-skewed population sex ratios in marine turtles based on future climate warming, using temperature recordings during incubation as a proxy for population demography (e.g. Hawkes et al., 2007; Fuentes et al., 2011; Laloë et al., 2014). Such sex ratio estimates, however, depend on the proxy used in sex determination, and may differ substantially when different proxies are used (Fuentes et al., 2011).
Recent advances in the sexing of marine turtles, which combine laparoscopic and endocrinological techniques, have, for the first time, provided empirical evidence for the impact of recent warming on contemporary marine turtle populations. Robust sex analysis enabled the researchers to link male and female green sea turtles to their natal beaches, leading to the discovery that northern (warmer) Great Barrier Reef populations have become highly female biased. Over 99% of juveniles and sub-adults originating from northern sites are female (compared to 67% female at southern sites) (Jensen et al., 2018). Despite the ability of marine turtles to maintain population viability even at highly female-biased sex ratios, a subadult female bias of 99.8% in northern green sea turtle populations indicates that the complete feminization, and thus local extinction, of northern GBR populations is possible in the near future (Jensen et al., 2018).

Gendered Innovation 2: Understanding Environmental Sex Determination beyond Temperature
Sex determination mechanisms fall on a continuum from environmental (ESD) to genetic (GSD). Primary sex differentiation has been shown to respond to a diverse range of environmental factors in a growing number of species. Hypoxia, for example, results in a higher ratio of males in zebrafish (Shang et al., 2006). Ocean acidification, in contrast, results in 16% more female oysters over a single generational cycle (Parker et al., 2018). Similarly, increased aquatic pH results in more female cichlids (Oldfield, 2005). What is increasingly apparent is that alterations in sex ratio—in either direction—will result in populations less resilient to further disturbance and potentially lead to demographic collapse. A mechanistic understanding of sex-change processes and other ecologically significant sex-based responses is thus vital to accurately modelling impacts of anthropogenic disturbance (e.g. overfishing or climate change) at a population level.
Gendered Innovation 3: Sex Analysis Enhances Effective Population Management
An accurate understanding of population structures enhances effective ecosystem management. Recent advances in next-generation sequencing technology has facilitated research focused on the causes and consequences of changes in population structure in a wide range of non-model species (Narum et al., 2013). However, only 9.6% of studies on marine and diadromous (tolerant of both seawater and freshwater) species have reported sex information (Benestan et al., 2017).
Benestan and colleagues (2017) demonstrate the importance of understanding sex ratios when employing sequencing to interpret population structure. In the American lobster, for example, these authors demonstrate how two populations (inshore and offshore) can be misunderstood as two distinct subpopulations if the sex ratio of samples is unintentionally biased. Studies that sample correctly, i.e. take care to collect equal numbers of males and females, show that in reality inshore and offshore American lobsters are a connected population that is mixed genetically. Omitting sex information in this instance leads to the incorrect classification of two distinct populations, which in turn may result in ineffective management recommendations.
The American lobster is the most valuable catch in North America. Effective population management has been demonstrated to successfully maximize its sustainable exploitation. Proactive harvester-driven conservation of commercial stocks in the Gulf of Maine, for example, has resulted in record hauls, despite warming (Le Bris et al., 2018). Conversely, in the absence of such management, warming has led to the collapse of the fishery in southern New England (Le Bris et al., 2018).
Accurate population demographic data is especially important when conservation efforts are sex biased. Lobster conservation efforts have protected large berried females (females carrying fertilized eggs on the underside of the abdomen), but not large males. This female-biased protection leads to selective harvesting of large males, which subsequently restricts mate choice and limits sperm, resulting in reduced reproductive success (Tang et al., 2019). In the face of climate change, effective management of this natural resource will become increasingly critical to preserve the reproductive potential of lobster stocks. Robust sex analysis will ensure population structure is understood, and thus managed and protected accurately.
Gendered Innovation 4: Understanding the Differential Sensitivity of Female and Male Marine Organisms to Climate Change
Anthropogenically induced climate change results in rising seawater temperatures, ocean acidification, hypoxic zones and storm events. Where tested, sex analysis has emerged as a key parameter for determining species responses to these environmental challenges. Elevated seawater pCO2 (dissolved carbon dioxide), for example, results in differential mortality between the sexes in Crustacea. Both female shrimp (Kurihara et al., 2008) and copepods (Cripps et al., 2014) show greater sensitivity to climate change than their male counterparts.
This pattern, however, does not always hold and analyses must be done carefully. In the copepod Arcatia tonsa, females show greater tolerance of elevated temperatures (Sasaki et al., 2019). Failing to account for sex differences in response to elevated temperatures risks underestimating population-level impacts by overlooking population decline caused by sperm limitation from greater male temperature sensitivity.
Sex differences in response to climate change can be complex. A recent study of copepod response to elevated pCO2 by Cripps and colleagues (2016) demonstrated this complexity. Under normal conditions, males have a higher baseline metabolic rate than females. In response to elevated pCO2, however, females increase and males decrease their metabolic rate (Cripps et al., 2016). Omitting sex analysis in this case masks sex-specific responses in physiological performance that are fundamental for determining population responses.
The differential sensitivity of female and male organisms to changing environmental conditions can also have a large, indirect and often unexpected impact on experimental outcomes. In marine copepods, for example, environmental conditions influence where individuals aggregate in the water column. Studies have shown that differential energy and reproductive requirements lead females to aggregate primarily in oxygenated surface layers of the water; males cluster in deeper water where oxygen concentrations are lower, as their location is primarily driven by temperature (Pierson et al., 2017). If this behavior is overlooked, researchers collecting specimens from a single depth would inadvertently introduce a sex ratio bias that would yield incorrect experimental conclusions.
Conclusions
Incorporating sex analysis throughout marine and environmental science innovation will facilitate discovery, research efficiency, reproducibility and robustness. Recent advances in sexing technologies will enable wider inclusion of sex analysis throughout these disciplines and facilitate better modelling of demographic change among marine organisms. This, in turn, will enhance our ability to effectively manage ecosystems and set conservation priorities.
Next Steps
To advance sex analysis in marine science and enhance its incorporation across the discipline, we recommend:
-
1. Policy developments in funding agencies and peer-reviewed journals that require sex analysis in marine science, and more broadly across the life sciences.
2. Development of novel sexing techniques for an increasingly wide array of non-model marine organisms, especially for species where morphological sexual dimorphism is not conspicuous.
3. Development of sexing techniques to improve accessibility, reliability, cost and ease. Increasing affordability of omics approaches and advancement of computing power will enable this endeavor.
4. Development of research that considers the role of hermaphroditism in sex disaggregated reporting of results (see Analyzing Sex in Hermaphroditic Species). This will enable better modelling of the timing, direction and duration of sex change, as well as sex determination mechanisms. This research should also consider individual responses to environmental stress where cells/behaviors/physiologies of different sexes co-exist simultaneously.
5. Modelling of ecosystem functioning and responses to climate change that incorporates sex analysis, where sex disaggregated data of sufficient quality is available. Such an endeavor will improve conservation efforts and MPA designation/management, as well as enable better management of fisheries.
Works Cited
Ainsworth, C. (2015). Sex redefined. Nature News, 518(7539), 288.
Bachtrog, D., Mank, J. E., Peichel, C. L., Kirkpatrick, M., Otto, S. P., Ashman, T. L., …& Perrin, N. (2014). Sex determination: why so many ways of doing it? PLoS biology, 12(7). https://doi.org/10.1371/journal.pbio.1001899
Benestan, L., Moore, J. S., Sutherland, B. J., Le Luyer, J., Maaroufi, H., Rougeux, C., … & Tallman, R .F (2017). Sex matters in massive parallel sequencing: evidence for biases in genetic parameter estimation and investigation of sex determination systems. Molecular ecology, 26(24), 6767-6783.
Cripps, G., Lindeque, P., & Flynn, K. J. (2014). Have we been underestimating the effects of ocean acidification in zooplankton? Global Change Biology 20, 3377-3385.
Cripps, G., Flynn, K. J., & Lindeque, P. K. (2016). Ocean acidification affects the phyto-zoo plankton trophic transfer efficiency. PLoS One 11. https://doi.org/10.1371/journal.pone.0151739
Diffenbaugh, N. S., & Field, C. B. (2013). Changes in ecologically critical terrestrial climate conditions. Science, 341(6145), 486-492.
Ellis, R. P., Davison, W., Queirós, A. M., Kroeker, K. J., Calosi, P., Dupont, S., …. & Urbina, M.A. (2017). Does sex really matter? Explaining intraspecies variation in ocean acidification responses. Biology letters, 13(2). https://doi.org/10.1098/rsbl.2016.0761
Ellis, R. P., Spicer, J. I., Byrne, J. J., Sommer, U., Viant, M. R., White, D. A., & Widdicombe, S., 2014. 1H NMR metabolomics reveals contrasting response by male and female mussels exposed to reduced seawater pH, increased temperature, and a pathogen. Environmental science & technology, 48(12), 7044-7052.
Fuentes, M. M. P. B., Limpus, C. J., & Hamann, M. (2011). Vulnerability of sea turtle nesting grounds to climate change. Global Change Biology, 17(1), 140-153.
Hawkes, L. A., Broderick, A. C., Godfrey, M. H., & Godley, B. J. (2007). Investigating the potential impacts of climate change on a marine turtle population. Global Change Biology, 13(5), 923-932.
Hawkes, L. A., Broderick, A. C., Godfrey, M. H., & Godley, B. J. (2009). Climate change and marine turtles. Endangered Species Research, 7(2), 137-154.
Hines, A., Yeung, W. H., Craft, J., Brown, M., Kennedy, J., Bignell, J., …. & Viant, M. R. (2007). Comparison of histological, genetic, metabolomics, and lipid-based methods for sex determination in marine mussels. Analytical Biochemistry, 369(2), 175-186.
Hoegh-Guldberg, O., & Bruno, J. F. (2010). The impact of climate change on the world’s marine ecosystems. Science, 328(5985), 1523-1528.
Jensen, M. P., Allen, C. D., Eguchi, T., Bell, I. P., LaCasella, E. L., Hilton, W. A., …. & Dutton, P. H. (2018). Environmental warming and feminization of one of the largest sea turtle populations in the world. Current Biology, 28(1), 154-159.
Jarne, P., & Auld, J.R. (2006). Animals mix it up too: the distribution of self‐fertilization among hermaphroditic animals. Evolution, 60(9), 1816-1824.
Kurihara, H., Matsui, M., Furukawa, H., Hayashi, M., & Ishimatsu, A. (2008). Long-term effects of predicted future seawater CO2 conditions on the survival and growth of the marine shrimp Palaemon pacificus. Journal of Experimental Marine Biology and Ecology 367, 41-46.
Laloë, J. O., Cozens, J., Renom, B., Taxonera, A., & Hays, G. C. (2014). Effects of rising temperature on the viability of an important sea turtle rookery. Nature Climate Change, 4(6), 513.
Le Bris, A., Mills, K. E., Wahle, R. A., Chen, Y., Alexander, M. A., Allyn, A. J., …. & Pershing, A. J. (2018). Climate vulnerability and resilience in the most valuable North American fishery. Proceedings of the National Academy of Sciences, 115(8), 1831-1836.
Mitchell, N. J., & Janzen, F. J. (2010). Temperature-dependent sex determination and contemporary climate change. Sexual Development, 4(1-2), 129-140.
Munday, P. L., Buston, P. M., & Warner, R. R. (2006). Diversity and flexibility of sex-change strategies in animals. Trends in Ecology & Evolution, 21(2), 89-95.
Narum, S. R., Buerkle, C. A., Davey, J. W., Miller, M. R., & Hohenlohe, P. A. (2013). Genotyping‐by‐sequencing in ecological and conservation genomics. Molecular ecology, 22(11), 2841-2847.
Oldfield, R. G. (2005). Genetic, abiotic and social influences on sex differentiation and the evolution of sequential hermaphroditism. Fish & Fisheries. 6, 93-110.
O'Leary, J. K., Micheli, F., Airoldi, L., Boch, C., De Leo, G., Elahi, R., …. & Lummis, S. (2017). The resilience of marine ecosystems to climatic disturbances. BioScience, 67(3), 208-220.
Ospina-Alvarez, N., & Piferrer, F. (2008). Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PloS one, 3(7). https://doi.org/10.1371/journal.pone.0002837
Pierson, J. J., Slater, W. C. L., Elliott, D., & Roman, M. R. (2017). Synergistic effects of seasonal deoxygenation and temperature truncate copepod vertical migration and distribution. Marine Ecology Progress Series, 575, 57-68.
Parker, L. M., O'Connor, W. A., Byrne, M., Dove, M., Coleman, R. A., Pörtner, H. O., …. & Ross, P.M., 2018. Ocean acidification but not warming alters sex determination in the Sydney rock oyster, Saccostrea glomerata. Proceedings of the Royal Society B: Biological Sciences, 285(1872), p.20172869.
Patrício, A. R., Varela, M. R., Barbosa, C., Broderick, A. C., Catry, P., Hawkes, L. A., …. & Godley, B. J. (2019). Climate change resilience of a globally important sea turtle nesting population. Global change biology, 25(2), 522-535.
Renner, S. S., & Ricklefs, R. E. (1995). Dioecy and its correlates in the flowering plants. American Journal of Botany, 82(5), 596-606.
Sasaki, M., Hedberg, S., Richardson, K., & Dam, H. G. (2019). Complex interactions between local adaptation, phenotypic plasticity and sex affect vulnerability to warming in a widespread marine copepod. Royal Society Open Science, 6(3). https://doi.org/10.1098/rsos.182115
Shang, E. H., Yu, R. M., & Wu, R. S. (2006). Hypoxia affects sex differentiation and development, leading to a male-dominated population in zebrafish. Environmental Science & Technology, 40(9), 3118-3122.
Tang, F., Sainte-Marie, B., Gaudette, J., & Rochette, R. (2019). Role of gamete limitation in the occurrence of ‘abnormal early clutches’ on female American lobster, Homarus americanus, in eastern Canada. Marine Biology, 166(11), 146.
Tannenbaum, C., Ellis, R. P., Eyssel, F., Zou, J., & Schiebinger, L. (2019). Sex and gender analysis improves science and engineering. Nature, 575(7781), 137-146.