Sex & Gender Analysis
Case Studies
- Science
- Health & Medicine
- Chronic Pain
- Colorectal Cancer
- Covid-19
- De-Gendering the Knee
- Dietary Assessment Method
- Gendered-Related Variables
- Heart Disease in Diverse Populations
- Medical Technology
- Nanomedicine
- Nanotechnology-Based Screening for HPV
- Nutrigenomics
- Osteoporosis Research in Men
- Prescription Drugs
- Systems Biology
- Engineering
- Assistive Technologies for the Elderly
- Domestic Robots
- Extended Virtual Reality
- Facial Recognition
- Gendering Social Robots
- Haptic Technology
- HIV Microbicides
- Inclusive Crash Test Dummies
- Human Thorax Model
- Machine Learning
- Machine Translation
- Making Machines Talk
- Medical Technologies
- Video Games
- Virtual Assistants and Chatbots
- Environment

Animal Research: Designing Health & Biomedical Research
The Challenge
Most basic research with animal models focuses on males and excludes females (Zucker et al., 2010; Marts et al., 2004). This creates three problems:
1. Less knowledge about disease processes in females due to underutilization of female animals. Results of studies in males are often generalized to females without justification, and even some conditions that occur more often in women are studied in mostly male animals. A gap exists between the proportion of women in patient populations and the proportion of female animals used in testing.
2. Inability to use sex as a variable in studies of basic biology (Holdcroft, 2007). In many cases, sex has proven an important variable—for example, in regulation of immune function.
3. Missed opportunities to examine female-specific phenomena (such as pregnancy and, in some species, menopause) that often interact with disease progression. Studying pregnancy in model organisms is especially important given the safety concerns about testing in pregnant women.
Method: Analyzing Sex in Lab Animal Research
Countries typically have enacted legislation that requires inclusion of women in government-sponsored human studies. For example, the U.S. National Institutes of Health require “that women and members of minorities and their subpopulations” be included in all human subjects research (although sufficient representation of women to allow for sex analysis is required only for Phase III clinical trials—see Policy Timeline). These guidelines, however, rarely apply to studies conducted on animals even though sampling animals of both sexes and of various hormonal states has produced new discoveries that influence drug development and patient care.
Gendered Innovations:
1. Studying sex differences in animal models has led to new treatments for traumatic brain injury (TBI), is more common in men than women.
2. Accounting for pregnancy, estrous cycle, and menopausal status in animal models has revealed the biological influence of hormones on basic molecular pathways and has been important to understanding certain autoimmune diseases.
3. Regulators have considered sex in order to improve animal models for toxicity; this has led to stronger environmental health standards.
The Underrepresentation of Female Animals
Gendered Innovation 1: Studying Sex leads to New Treatments for Traumatic Brain Injury
Method: Analyzing Sex
Method: Designing Studies in Biomedical Research
Gendered Innovation 2: Sampling with Attention to Estrous Cycles and Menopause Advances Basic Knowledge of the Immune System
Gendered Innovation 3: Strengthening Environmental Health Standards
Method: Rethinking Standards and Reference Models
Standards and Reference Models in the EU
Standards and Reference Models in the U.S.
Conclusions
Next Steps
The Challenge
Research using animals has been vital to Western science and medicine since its inception. Until the 1960s, however, the sex of animals used in research was rarely reported except in experiments related to reproduction. Even today, the sex of animal subjects is “omitted in 22–42% of articles in neuroscience, physiology, and interdisciplinary biology journals” (Beery et al., 2011).
Analysis of animal studies in which sex is reported shows that females are underrepresented in most subfields except reproductive biology and immunology—see chart below. 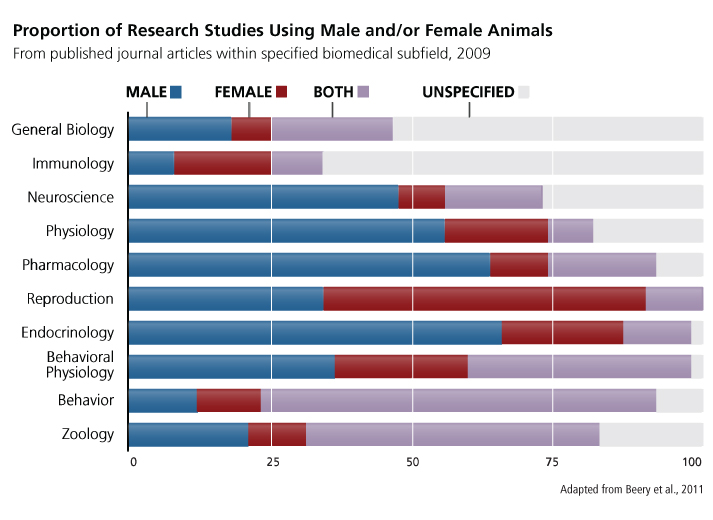
The Underrepresentation of Female Animals
Researchers may perform single-sex animal studies to reduce the cost of experiments or in hopes of lowering the variance of results (McCarthy et al., 2002). Single-sex experiments are the only option in studying sex-specific phenomena (ovarian cancer or prostate cancer, for instance) and can also be beneficial if one sex has been understudied or if there is strong evidence that sex does not influence outcome. The majority of single-sex animal experiments, however, do not fall into these categories. Female animals are underrepresented in studies of conditions that affect both sexes and are underrepresented in research where evidence suggests that sex influences outcome.
Researchers may avoid using female animals because hormone levels, which fluctuate throughout the estrous cycle, can interact with experimental outcomes (Becker et al., 2005; Wizemann et al., 2001). More rarely, researchers may avoid using male animals because, in some species and strains, inter-male aggression makes caging difficult (Gatewood et al., 2006). Female rodents may be preferred in toxicology studies because of their greater sensitivity to some toxins (European Commission, 2008).
Gendered Innovation 1: Studying Sex leads to New Treatments for Traumatic Brain Injury
Traumatic brain injury (TBI) is more common in men than in women both in Europe (where the leading cause is motor vehicle collisions) and in the United States (where the leading cause is firearm injuries; Tagliaferri et al., 2006; Wagner et al., 2000; Roof et al., 2000). New studies of TBI that include female animals have allowed sophisticated sex analysis and produced innovations in treatment for TBI patients (see Method).
Method: Analyzing Sex
Sex analysis begins when researchers use animals of both sexes in an experiment and analyze data to determine whether outcomes for females and males are different. In animal models of TBI, females consistently exhibit better outcomes than males—that is, females are the “most protected” sex and males are the “most affected.” This difference holds across multiple species and a variety of inbred and outbred mouse strains (Hurn et al., 2005).
Once a sex difference is observed, further experiments can elucidate the mechanism of difference (Grove et al., 2010)—see Method below.
Method: Analyzing Sex in Lab Animal Research
Determining the presence or absence of a sex difference requires consideration of the estrous cycles of female animals; if the estrous cycle is not considered, sex differences might exist but not be detected, as a result of averaging over the cycle (Stoffel et al., 2003). Mechanistic studies are also needed to definitively assess sex differences or lack thereof in animal experiments; for example, a particular drug or other intervention might produce the same effects in both sexes but act by different mechanisms (Liu et al., 2007). Furthermore, multiple sex-specific differences can have opposing effects and cancel each other out, preventing observation (Palaszynski et al., 2005).
If a sex difference is observed in an animal model, it is important to test for the contribution of sex hormones. Testing may include:
1. Sampling Female Animals at Different Points in the Estrous Cycle. In reproductively competent animals, a powerful study design involves monitoring female animals’ estrous cycles. A basic experimental design might involve ten groups of mice: two groups of males (experimental and control) and eight groups of females (experimental and control for each of the four days of the estrous cycle). A simpler option is to include females representing only two parts of the cycle, typically estrus and diestrus (Becker et al., 2005). Furthermore, as some mammals exhibit synchrony of ovulation, females should be housed to prevent close contact between mice ovulating on different days (Meziane et al., 2007).
In animal models of TBI, estrous cycle appears to have little influence on outcome (Wagner et al., 2004). But estrous cycle effects have been important in studies of immune function (see Gendered Innovation 2 below).
2. Sampling Female Animals during Pregnancy or Pseudopregnancy. TBI researchers who sampled male rats, normally cycling female rats, and pseudopregnant female rats found that edema was most severe in males, less severe in normally cycling females, and least severe in pseudopregnant females (Roof et al., 1993). Progesterone levels are known to be lowest in males, higher in normally cycling females, and highest in pregnant or pseudopregnant females, which suggested to researchers that progesterone may protect against edema (Meffre et al., 2007).
3. Artificially Manipulating Hormones. In animal models of TBI, ovariectomy reduces the survival advantage that intact females have over males. Injection of progesterone partially restores this advantage in ovariectomized females and also improves survival in males (Bayir et al., 2004).
Researchers used evidence from animal models of TBI—obtained through analyzing sex and sampling female animals in different hormonal states—to devise an experimental treatment for humans. In double-blind clinical studies, patients who received progesterone shortly after emergency treatment for TBI had lower mortality and showed better recovery of neurological function than control patients with similar injury, and progesterone was well-tolerated (Xiao et al., 2008; Wright et al., 2007). More research is needed to:
• Evaluate risks and benefits of progesterone treatment according to patient characteristics (such as sex and age) and injury characteristics.
• Elucidate the mechanisms by which progesterone protects against brain damage in TBI.
Gendered Innovation 2: Sampling with Attention to Estrous Cycles and Menopause Advances Basic Knowledge of the Immune System
By including female mice in experiments, scientists discovered that sex hormones are important to immune system function. When female mice were exposed to antigens and then sampled during diestrus or estrus, their immune responses in the spleen were similar to those seen in males. But when female mice were sampled during proestrus or metestrus, their antibody counts were more than triple that of males (Krzych et al., 1978). By correlating these differences with progesterone and estrogen concentrations (which also vary throughout the estrous cycle), it was possible to uncover the influence of sex hormones on immune function (Bergman et al., 1992).
Animal models of menopause—which are still in development (see Next Steps below)—have shown that immunological changes accompany this hormonal transition. When mice are ovariectomized and undergo "acute menopause," they exhibit "reduced lymphocyte chemotaxis, mitogen-induced T cell proliferation responses, and [Interleukin-2] production" (Marriott et al., 2006).
An understanding of hormones and immune function is relevant to treating numerous diseases, including autoimmune diseases and HIV infection. For example, animal models have been used to investigate why HIV viral load tends to increase more rapidly in men than in women (Meier et al., 2009).
Gendered Innovation 3: Strengthening Environmental Health Standards
In addition to their use in basic research and preclinical testing, animal models are integral to environmental monitoring and evaluating the toxicity of chemicals. The European Commission's Institute for Health and Consumer Protection (IHCP) and the U.S. National Toxicology Program (NTP) have analyzed reference models to improve environmental standards (see Method).
Method: Rethinking Standards and Reference Models
Consideration of sex in toxicology reference models is important: Both model organisms and humans show sex differences in sensitivity to certain toxins, and in some cases, a compound may have qualitatively different effects in females and males—particularly if the compound is an endocrine disruptor (see Case Study: Environmental Chemicals).
The IHCP uses a "sex-linked recessive lethal test" in drosophila as a model of mutation in order to screen chemicals for mutagenic activity (European Commission, 2008).
Standards and Reference Models in the EU
The European Commission’s IHCP has strong requirements for sex analysis and sampling (European Commission, 2008):
1. Inclusion of Female and Male Animals. For example, in inhalation toxicity testing, researchers are instructed to use equal numbers of females and males at each concentration level. In other tests, the sex more sensitive to a particular toxin, generally females, is preferred.
2. Reporting the Sex of Study Subjects. Regardless of whether an experiment is single-sex or mixed-sex, the IHCP requires reporting “number, age, and sex of animals”.
3. Sampling Pregnant Females to Detect Developmental Toxicity. This protocol allows researchers to gather “information concerning the effects of prenatal exposure on the pregnant test animal and on the developing organism in utero”.
Standards and Reference Models in the U.S.
The U.S. National Toxicology Program (NTP) requires sex analysis in all routine animal toxicity studies (NTP, 2006), which must:
1. Report the Sex of Study Subjects. NTP states that “data are to be tabulated and organized by species, sex, and treatment group.”
2. Analyze Results by Sex and Report Null Findings. All analyses performed by the NTP note the presence, absence, and statistical significance of sex differences.
3. Analyze Factors Intersecting with Sex. All analyses are to be controlled for weight so that weight differences are not misreported as sex differences or vice versa.
Conclusions
Gendered innovations:
1. Physiology and Pathophysiology: Including female animals in experimental studies led to new knowledge about traumatic brain injury, resulting in new therapeutics. Sampling with regard to sex and hormonal state has also produced knowledge about hormonal regulation of the immune system, relevant to the treatment of autoimmune diseases and infections. This information is now being applied to development of treatment dosing for vaccines (Klein et al., 2010; World Health Organization, 2010).
2. Regulatory Policy: Analyzing sex has become a critical part of both EU and U.S. efforts to understand, prevent, and control environmental pollution.
Next Steps
A. Future research needs include:
1. Analyzing Sex at the Tissue and Cellular Level. Sex analysis in basic research has occurred primarily in animal studies and has centered on hormonally mediated sex differences. Sex is rarely analyzed or even reported in studies involving cultured cells or extracted tissues. A study of articles in high-impact, peer-reviewed cardiovascular disease journals showed that only 20-28% of articles describing research on new cell lines stated the sex of cells used. Of the minority of studies that did report sex, 69% used male cells only (Taylor et al., 2011). This disparity is of concern because emerging research suggests that studying cellular sex is important in developing stem cell therapies (see Case Study: Stem Cells).
2. Analyzing Factors Intersecting with Sex to Avoid Overemphasizing Sex Differences. Not all observed differences between female and male animals, cells, or tissues—or between women and men—are due to biological sex. Analyzing factors intersecting with sex and gender is critical to avoid overemphasizing sex differences. Important factors include diet, hormone levels, and species. Maternal interactions shortly after birth contribute to sex differences in behavior: Mother rats interact differently with female and male pups, producing developmental differences (Moore, 1992).
3. Developing Animal Models of Menopause. High-quality, validated animal models of menopause are needed. Although primates undergo menopause-like processes, the challenges of using primates and the scarcity of older female animals limit research. Menopause can be induced surgically in experimental animals through ovariectomy, which models bilateral oophorectomy in women but may not be comparable to natural human menopause (Bellino et al., 2003). Strategies for modeling human menopause in rodents include treating mice or rats with drugs that induce premature ovarian failure and using transgenic mice (such as strain Foxo3a-/-) that show accelerated ovarian senescence (Wu et al., 2005).
4. Studying Gender in Animal Research. Placing female and male animals in different physical and social environments can have marked effects on behavior and experimental outcome, and gender analysis is needed to ensure that housing systems and handling do not create systematic bias (Holdcroft, 2007). In particular, if researchers expect a particular sex difference, they may handle or house female and male animals differently and in such a manner as to produce that sex difference, or they may choose a specific behavioral test likely to produce that difference (Birke, 2011). Housing and handling can determine animal stress levels, which alter both behavioral and biochemical profiles (Beck et al., 2002).
B. Policy next steps include:
1. Requiring Researchers to Report the Sex of Subjects. Granting agencies and journal editors can make sex reporting a requirement if research is to be funded or findings published. Reporting the sex of model organisms prevents inappropriate generalizations, facilitates meta-analysis, and can show where animals of one sex have been overlooked. Major bioscience funders, including the U.K.’s Medical Research Council (MRC), now require that researchers report animal “species, strain, sex, developmental stage […] and weight.” Major journals, including Nature and the Public Library of Science publications, have instituted the same requirements (National Centre for the Replacement, Refinement, and Reduction of Animals in Research, 2008; Kilkenny et al., 2010).
2. Requiring Two-Sex Studies and Sex Analysis. Government agencies can require that, where appropriate, publically funded studies include animals of both sexes and be designed with “adequate sample sizes” for each sex. Public and private funders alike can “treat inclusion of female animals as a matter of scientific merit that affects funding” (Beery et al., 2011).
3. Standardizing the Use of “Sex” and “Gender” in Relation to Animal Research. Currently, the terms “sex” and “gender” are used interchangeably in much animal research, complicating literature searches and meta-analysis. Sex and gender are not interchangeable. Standardizing usage, and using “sex” to refer to the biological trait of femaleness or maleness, would remedy this problem (Marts, 2004).
Works Cited
Bayir, H., Marion, D., Puccio, A., Wisniewski, S., Janesko, K., Clark, R., & Kochanek, P. (2004). Marked Gender Effect on Lipid Peroxidation after Severe Traumatic Brain Injury in Adult Patients. Journal of Neurotrauma, 21 (1), 1-8.
Becker, J., Arnold, A., Berkley, K., Blaustein, J., Eckel, L., Hampson, E., Herman, J., Marts, S., Sadee, W.,
Steiner, M., Taylor, J., & Young, E. (2005). Strategies and Methods for Research on Sex Differences in Brain and Behavior. Endocrinology, 146 (4), 1650-1673.
Beck, K., & Luine, V. (2002). Sex Differences in Behavioral and Neurochemical Profiles after Chronic Stress: Role of Housing Conditions. Physiology and Behavior, 75 (5), 661-673.
Beery, A., & Zucker, I. (2011). Sex Bias in Neuroscience and Biomedical Research. Neuroscience and Biobehavioral Reviews, 35 (3), 565-572.
Bellino, F., & Wise, P. (2003). Nonhuman Primate Models of Menopause Workshop. Biology of Reproduction, 68 (1), 10-18.
Bergman, M., Schachter, B., Karelus, K., Combatsiaris, E., Garcia, T., & Nelson, J. (1992). Up-Regulation of the Uterine Estrogen Receptor and its Messenger Ribonucleic Acid during the Mouse Estrus Cycle: The Role of Estradiol. Endocrinology, 130 (4), 1923-1930.
Birke, L. (2011). Telling the Rat What to Do: Laboratory Animals, Science, and Gender. In Fisher, J. (ed.), Gender and the Science of Difference: Cultural Politics of Contemporary Science and Medicine, pp. 91-107. New Brunswick: Rutgers University Press.
European Commission. (2008). Council Regulation EC-440-2008: Laying Down Test Methods Pursuant to Regulation EC-1907-2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH). Official Journal of the European Union, 31 (5), 142-739.
Gatewood, J., Wills, A., Shetty, S., Xu, J., Arnold, A., Burgoyne, P., & Rissman, E. (2006). Sex Chromosome
Complement and Gonadal Sex Influence Aggressive and Parental Behaviors in Mice. The Journal of Neuroscience, 26 (8), 1335-2342.
Grove, K., Fried, S., Greenberg, A., Xiao, Z., & Clegg, D. (2010). A Microarray Analysis of Sexual Dimorphism of Adipose Tissue in High-Fat-Diet-Induced Obese Mice. International Journal of Obesity, 34, 989-1000.
Holdcroft, A. (2007). Integrating the Dimensions of Sex and Gender into Basic Life Sciences Research: Methodologic and Ethical Issues. Gender Medicine, 4 (S2), S64-S74.
Hurn, P., Vannucci, S., & Hagberg, H. (2005). Adult or Perinatal Brain Injury : Does Sex Matter? Stroke, 36 (2), 193-195.
Kilkenny, C., Browne, W., Cuthill, I., Emerson, M., & Altman, D. (2010). Animal Research: Reporting In Vivo Experiments. Public Library of Science (PLoS) Biology, 8 (6), e1000412.
Klein, S., Passaretti, C., Anker, M., Olukoya, P., & Pekosz, A. (2010). The Impact of Sex, Gender, and Pregnancy on 2009 H1N1 Disease. Biology of Sex Differences, 1 (5), 1-12.
Krzych, U., Strausser, H., Bressler, J., & Goldstein, A. (1978). Quantitative Differences in Immune Responses during
the Various Stages of the Estrus Cycle in Female BALB/c Mice. The Journal of Immunology, 121 (4), 1603-1605.
Liu, N., von Gizycki, H., & Gintzler, A. (2007). Sexually Dimorphic Recruitment of Spinal Opioid Analgesic Pathways by the Spinal Application of Morphine. Journal of Pharmacology and Experimental Therapeutics, 322 (2), 654-660.
Marriott, I., & Huet-Hudson, Y. (2006). Sexual Dimorphism in Innate Immune Responses to Infectious Organisms. Immunologic Research, 34 (3), 177-192.
Marts, S., & Keitt, S. (2004). Foreword: A Historical Overview of Advocacy for Research in Sex-Based Biology. Advances in Molecular and Cell Biology, 34, V-XIII.
McCarthy, M., & Becker, J. (2002). Neuroendocrinology of Sexual Behavior in the Female. In Becker, J., Breedlove, S., Crews, D., & McCarthy, M. (Eds.), Behavioral Endocrinology, pp. 124-132. Massachusetts: MIT Press.
Meffre, D., Pianos, A., Liere, P., Eychenne, B., Cambourg, A., Schumacher, M., Stein, D., & Guennoun, R. (2007). Steroid Profiling in Brain and Plasma of Male and Pseudopregnant Female Rats after Traumatic Brain Injury: Analysis by Gas Chromatography / Mass Spectrometry. Endocrinology, 148 (5), 2505-2517.
Meier, A., Chang, J., Chan, E., Pollard, R., Sidhu, H., Kulkarni, S., Wen, T., Lindsay, R., Orellana, L., Mildvan, A., Bazner, S., Streeck, H., Alter, G., Lifson, J., Carrington, M., Bosch, R., Robbins, G., & Altfeld, M. (2009). Sex Differences in the Toll-Like Receptor—Mediated Response of Plasmacytoid Dendritic Cells to HIV-1. Nature Medicine, 15 (8), 955-959.
Meziane, H., Ougazzal, M., Aubert, L., Wietrzych, M., & Krezel, W. (2007). Estrus Cycle Effects on Behavior of C57BL/6J and BALB/cByJ Female Mice: Implications for Phenotyping Strategies. Genes, Brain and Behavior, 6 (2), 192-200.
Michael, S. (1976). Plasma Prolactin and Progesterone during the Estrus Cycle in the Mouse. Proceedings of the Society for Experimental Biology and Medicine, 153 (2), 254-257.
Moore, C. (1992). The Role of Maternal Stimulation in the Development of Sexual Behavior and its Neural Basis. Annals of the New York Academy of Sciences, 662, 160-177.
National Centre for the Replacement, Refinement, and Reduction of Animals in Research (NC3RS). (2008). Responsibility in the Use of Animals in Bioscience Research: Expectations of the Major Research Council and Charitable Funding Bodies. London: Tradewinds.
National Toxicology Program (NTP). (2006). Specifications for the Conduct of Studies to Evaluate the Toxic and Carcinogenic Potential of Chemical, Biological, and Physical Agents in Laboratory Animals for the NTP. Washington, D.C.: Government Publishing Office (GPO).
Palaszynski, K., Smith, D., Kamrava, S., Burgoyne, P., Arnold, A., & Voskuhl, R. (2005). A Yin-Yang Effect between Sex Chromosome Complement and Sex Hormones on the Immune Response. Endocrinology, 148 (6), 3280-3285.
Roof, R., Duvdevani, R., & Stein, D. (1993). Gender Influences Outcome of Brain Injury: Progesterone Plays a Protective Role. Brain Research, 607, 333-336.
Stoffel, E., Ulibarri, C., & Craft, R. (2003). Gonadal Steroid Hormone Modulation of Noiception, Morphine Antinoiception, and Reproductive Indices in Male and Female Rats. Pain, 103 (3), 285-302.
Tagliaferri, R., Compagnone, C., Korsic, M., Servadei, F., & Kraus, J. (2006). A Systematic Review of Brain Injury Epidemiology in Europe. Acta Neurochirurgica, 148, 255-268.
Taylor, K., Vallejo-Giraldo, C., Schaible, N., Zakeri, R., & Miller, V. (2011). Reporting of Sex as a Variable in Cardiovascular Studies using Cultured Cells. Biology of Sex Differences, 2 (11), 1-7.
Wagner, A., Willard, L., Kline, A., Wenger, M., Bolinger, B., Ren, D., Zafonte, R., & Dixon, C. (2004). Evaluation of Estrus Cycle Stage and Gender on Behavioral Outcome after Experimental Traumatic Brain Injury. Brain Research, 998 (1), 113-121.
Wagner, A., Sasser, H., Hammond, F., McConnell, F., Wiercisiewski, D., & Alexander, J. (2000). Intentional Traumatic Brain Injury: Epidemiology, Risk Factors, and Associations with Injury Severity and Mortality. Journal of Trauma, Injury, Infection, and Critical Care, 49 (3), 404-410.
Wald, C., & Wu, C. (2010). Of Mice and Women: The Bias in Animal Models. Science, 327 (5973), 1571-1572.
Wizemann, T., & Pardue, M. (2001). Exploring the Biological Contributions to Human Health: Does Sex Matter? Washington, D.C.: National Academies Press.
World Health Organization (WHO). (2010). Sex, Gender, and Influenza. Geneva: WHO Press.
Wright, D., Kellermann, A., Hertzberg, V., Clark, P., Frankel, M., Goldstein, F., Salomone, J., Dent, L., Harris, O., Ander, D., Lowery, D., Patel, M., Denson, D., Gordon, A., Wald, M., Gupta, S., Hoffman, S., & Stein, D. (2007). ProTECT: A Randomized Clinical Trial of Progesterone for Acute Traumatic Brain Injury. Annals of Emergency Medicine, 49 (4), 391-402.
Wu, J., Zelinski, M., Ingram, D., & Ottinger, M. (2005). Ovarian Aging and Menopause: Current Theories, Hypotheses, and Research Models. Experimental Biology and Medicine, 230 (11), 818-829.
Xiao, G., Wei, J., Yan, W., Wang, W., & Lu, Z. (2008). Improved Outcomes from the Administration of Progesterone for Patients with Acute Severe Traumatic Brain Injury: A Randomized Controlled Trial. Critical Care, 12 (2), 1-10.
Zucker, I., & Beery, A. (2010). Males Still Dominate Animal Studies. Nature Editorials, 465, 690.
Integrating Sex and Gender into Animal research image
Between 1997 and 2000, 10 drugs were withdrawn from the U.S. market because of life-threatening health effects. Eight of these posed “greater health risks for women than for men.” Not only did these drugs cost billions of dollars to develop, but when they failed, they caused death and human suffering. We can’t afford to get the research wrong.
One reason drugs fail—and fail more often for women—is that, oddly enough, most research is still done in males, whether human, animal, or cells and tissues (see chart).

In research design, both male and female animals should be considered before sex differences are ruled out. Although this increases costs in basic research, it may reduce costs overall—given the high price of developing biomedical therapies. It will certainly reduce human suffering and death.
Countries typically have legislation that requires inclusion of women in government-sponsored Phase III clinical trials. These guidelines, however, rarely apply to studies conducted in animals or cells and tissues. This means that drugs and therapies may be less effective for females and also that anything unique to females will not be seen in the discovery phase of research.
Gendered Innovations:
Sampling animals of both sexes and of various hormonal states has produced new discoveries that influence drug development and patient care.
- 1. Studying sex differences in animal models has led to new treatments for traumatic brain injury (TBI), which is more common in men than women.
- 2. Accounting for estrous cycle, pregnancy, and menopausal status in animal models has revealed the biological influence of hormones on basic molecular pathways and has been important to understanding certain autoimmune diseases.
- 3. Regulators have considered sex in order to improve animal models for toxicity; this has led to stronger environmental health standards.






